Abstract
Background
Efavirenz-based antiretroviral therapy (ART) regimens are preferred for treatment of adult HIV-positive patients co-infected with tuberculosis (HIV/TB). Few studies have compared outcomes among HIV/TB patients treated with efavirenz or non-efavirenz containing regimens.
Methods
HIV-positive patients aged ≥16 years with a diagnosis of tuberculosis recruited to the TB:HIV study between Jan 1, 2011, and Dec 31, 2013 in 19 countries in Eastern Europe (EE), Western Europe (WE), and Latin America (LA) who received ART concomitantly with TB treatment were included. Patients either received efavirenz-containing ART starting between 15 days prior to, during, or within 90 days after starting tuberculosis treatment, (efavirenz group), or other ART regimens (non-efavirenz group). Patients who started ART more than 90 days after initiation of TB treatment, or who experienced ART interruption of more than 15 days during TB treatment were excluded. We describe rates and factors associated with death, virological suppression, and loss to follow up at 12 months using univariate, multivariate Cox, and marginal structural models to compare the two groups of patients.
Results
Of 965 patients (647 receiving efavirenz-containing ART, and 318 a non-efavirenz regimen) 50% were from EE, 28% from WE, and 22% from LA. Among those not receiving efavirenz-containing ART, regimens mainly contained a ritonavir-boosted protease inhibitor (57%), or raltegravir (22%). At 12 months 1.4% of patients in WE had died, compared to 20% in EE: rates of virological suppression ranged from 21% in EE to 61% in WE. After adjusting for potential confounders, rates of death (adjusted Hazard Ratio; aHR, 95%CI: 1.13, 0.72–1.78), virological suppression (aHR, 95%CI: 0.97, 0.76–1.22), and loss to follow up (aHR, 95%CI: 1.17, 0.81–1.67), were similar in patients treated with efavirenz and non-efavirenz containing ART regimens.
Conclusion
In this large, prospective cohort, the response to ART varied significantly across geographical regions, whereas the ART regimen (efavirenz or non-efavirenz containing) did not impact on the proportion of patients who were virologically-suppressed, lost to follow up or dead at 12 months.
Similar content being viewed by others
Background
Globally, tuberculosis (TB) is the commonest opportunistic infection in HIV-infected patients, especially in resource-constrained settings [1]. Mortality in co-infected patients varies across regions as reported previously [2, 3], however, in most regions TB is the most common cause of death among the HIV-infected adult population [4,5,6]. Prospective cohort studies demonstrated that HIV patients co-infected with TB have an increased risk of death [5], and reduced mortality with appropriate TB treatment and initiation of antiretroviral therapy (ART) [7, 8]. Additionally, in patients with TB who present with advanced immunodeficiency (CD4 count < 200 cells/mm3), early initiation of ART (< 8 weeks after TB treatment initiation) increases survival, as demonstrated in several large clinical trials [9,10,11,12]. Efavirenz-based regimens were used in all of these studies [9,10,11,12]. The efficacy of other ART regimens in TB/HIV co-infected patients, and the relative efficacy of rifampicin- or rifabutin-containing regimens in patients receiving ART remains poorly studied [13,14,15]. Hence, National and regional guidelines provide minimal guidance on such ART regimens used concomitantly with tuberculosis treatment [1, 16,17,18,19,20,21,22].
On the other hand, late presentation of HIV-positive adult patients into care is common in both resource-constrained and high-income settings [23,24,25] and episodes of opportunistic infection including TB, may occur within the first few months after initiation of ART [8]. In most parts of the world with limited resources and a high burden of TB, efavirenz remains the preferred agent as the third component of ART regimens, as it has no clinically-significant interaction with rifampicin [1, 26]. However, tolerability and non-nucleoside reverse transcriptase inhibitor resistance may preclude use of efavirenz. In 2007, the WHO recommended increasing the ritonavir boosting dose to 400 mg every 12 h with lopinavir when concomitantly used in combination with rifampicin [21] however this has been associated with poor tolerability and hepatotoxicity [27,28,29]. More recently, raltegravir was reported to be an adequate alternative to efavirenz in TB/HIV co-infected patients in a phase 2 trial [30]. Therefore, CDC and WHO both indicate raltegravir to be the preferred option when efavirenz is contraindicated, or the use of rifabutin when ritonavir-boosted protease inhibitors are necessary [1, 16, 18, 31]. However, access to rifabutin and raltegravir in low- and middle-income countries, is often difficult or impossible [30]. The aim of this study was to assess clinical outcomes (mortality, virological suppression, loss to follow-up during the first year following TB diagnosis) across different regions (Eastern Europe, Western Europe, and Latin America) among HIV-positive adult patients treated with efavirenz and non-efavirenz containing ART regimens.
Methods
Study population
The TB:HIV study is a collaboration between TB and HIV clinicians from 19 countries in Europe and Latin America. Patients aged 16 years or older were included if they were HIV-positive and were diagnosed with TB between January 1, 2011, and December 31, 2013. Patients with confirmed TB (Mycobacterium tuberculosis on culture or PCR), probable TB (Acid-Fast Bacilli on ‘smear’ or granulomatous inflammation on biopsy specimens) and presumptive TB (empiric TB treatment initiated, and with a TB diagnosis not subsequently ruled out) were included in the study. Details of the TB:HIV study have been published elsewhere [2]. For the main analysis, those who initiated tuberculosis treatment and ART concomitantly were analyzed. Data on TB and HIV disease, including demographic, clinical, laboratory parameters, and clinical outcomes were collected prospectively on standardized case report forms (http://www.cphiv.dk). Due to the observational nature of the study, all decisions regarding use of tuberculosis and ART treatment regimens was at the discretion of individual clinicians.
Statistical analyses
In this study, patients were categorized into two groups according to their ART regimen. In the efavirenz group patients who initiated an efavirenz-containing ART regimen within 3 months of starting TB therapy, or who were receiving it (at least in the 2 weeks before TB diagnosis) were included. Patients, who interrupted ART for more than 15 days while on anti-TB drugs, were excluded. In the non-efavirenz group, patients receiving ART but not containing efavirenz within the above time frame were included. Patients in this group included those in receipt of ritonavir-boosted protease inhibitors, triple nucleoside reverse transcriptase inhibitors, integrase inhibitors or nevirapine. Baseline was considered the date anti-TB treatment was commenced. Demographic and clinical characteristics of patients at baseline were described by receipt of ART in each region and for all study participants. Patients were recorded as dead, in care with virological suppression, in care without virological suppression, in care without viral load information, or lost to follow up at 12 months after their TB diagnosis. A viral load < 400 copies/mL between 8 and 12 months after baseline was considered as virological suppression. Patients with a last visit date reported before 12 months of follow-up were considered lost to follow up. All patients were included in analysis of time to outcome, and were censored 12 months after the TB diagnosis.
The main outcome was mortality during the first year following TB diagnosis comparing efavirenz and non-efavirenz containing ART regimens, using three different statistical methods to account for potential confounders and bias: inverse probability weighting, Cox models and marginal structural models. For the first method, Kaplan-Meier survival curves were estimated using inverse weighting. Weights were calculated from a logistic model to predict efavirenz use, adjusting by traditional confounders: region, age, gender, CD4 count at TB diagnosis, type of TB, route of HIV transmission, naïve status, rifamycin use (rifampicin or rifabutin), and multidrug-resistant TB (MDR-TB). In the second method, a Cox model was used to estimate risk of death using the same co-variables, stratifying by naïve status. Continuous variables such as age and CD4 cell count were included in the models using splines with three knots to fit a non-linear and less biased relationship with the outcome [32]. Finally, a marginal structural model was used to estimate the effect of both ART regimes on mortality in the presence of varying time co-variables (time-dependent confounders), as well as type of rifamycin used, CD4 count, and resistance to anti-TB drugs (variables that could affect choice of ART regimen). With this method, simulation of a clinical trial comparing two regimes of ART in HIV individuals co-infected with TB, controlling by time updated measurements of the confounders, was attempted. To achieve this, a monthly data set was generated for each patient selected from the time of TB diagnosis until 12 months of follow-up, recording all the socio-demographic and clinical endpoints month by month.
Following standard use of marginal structural models, patients who did not receive ART at initiation of TB treatment were included in this analysis since, as they were on tuberculosis treatment, they had the potential to be started by clinicians on ART with any regimen, as in local clinical practice [33]. The dataset was expanded in order to have the same patients and their characteristics in either of two possible regimens of ART (efavirenz-containing, or not), and artificial censoring was created to keep only the time in which patients did not leave the regimen. To adjust for a potential bias due to unbalance of co-variables in each regimen stabilized weights from two logistic models were built to predict use of ART controlling by time, age, gender, region, naive status, rifamycin use, injection drug use (IDU) status, type of TB (disseminated compared to pulmonary), CD4 count, and MDR-TB. The probability of death was estimated with a pooled logistic regression model using the ART regimen, time in months, region, CD4 count, gender, naïve status, MDR-TB status, with rifamycin receipt as a co-variable and with stabilized weights [33]. Sensitivity analysis was done including only ART-naïve patients. Cox models adjusted by traditional confounders were used, looking for factors related to virological suppression among patients at 12 months after diagnosis of TB who were in care with a viral load available, and who were lost to follow up.
Results
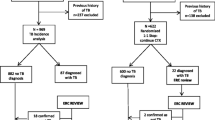
Of 1389 patients in the study, 965 (69%) received ART during the predefined time-frame and were included in the main analysis: 647 were in the efavirenz group and 318 in the non-efavirenz group. We excluded 177 patients because they started ART after the time-frame (i.e. > 3 months after initiation of TB treatment), and 18 because of ART interruptions (> 15 days) during TB treatment. Baseline characteristics of participants stratified by ART regimen and ART status are shown in Table 1. Patients were treated with a rifamycin (rifampin or rifabutin) for a median of 7 months (IQR: 2–10). Patients had a median of one viral load measurement during the first year following ART initiation (IQR: 0–2).
Clinical outcomes at 12 months
Mortality, the proportion of patients with undetectable viral loads, and loss to follow up was similar for patients receiving efavirenz and non-efavirenz ART regimens, irrespective of whether patients were ART-naïve or ART-experienced at TB diagnosis (Table 2). Clinical outcomes differed significantly across regions, although within regions, similar outcomes were observed for patients receiving efavirenz and non-efavirenz ART regimens (Fig. 1). The highest proportion of deaths and the lowest proportion of patients with undetectable HIV viral loads at 12 months were observed in EE. Many patients in all regions had no viral load information in the time window 8–12 months following TB diagnosis: 25% in EE, 17% in WE, and 23% in LA) for those receiving an efavirenz-containing regimen (p < 0.01), and 32%, 15% and 25% for those taking a non-efavirenz regimen, respectively (p < 0.01) (Fig. 1).
Survival
By 12 months 113(12%) deaths had occurred: 84(13%) in the efavirenz group and 29(9%) in the non-efavirenz group. Using an inverse probability weighting method, no difference in survival (adjusted by region, age, gender, CD4 count at TB diagnosis, type of TB, route of HIV transmission, naïve status, rifamycin use at TB diagnosis, and MDR-TB) was observed between efavirenz and non-efavirenz treated individuals (Additional file 1: Figure S1). In adjusted Cox regression models stratified by ART status and use of rifamycin, the hazard ratio (HR) for death was 1.13 (95% CI: 0.72–1.78; p = 0.59) for efavirenz compared with non-efavirenz ART regimens (Table 3). In the same model, higher CD4 counts at TB diagnosis were associated with a lower risk of death (HR 0.27, 95%CI: 0.17–0.42, p < 0.001) for patients with CD4 of 350 cells/mm3 compared to those with CD4 50 cells/mm3, and naïve patients had a lower risk of death (HR 0.64, 95%CI: 0.43–0.96, p = 0.03) (Table 3). Gender, age, route of HIV transmission, disseminated TB, and MDR-TB were not significantly associated with mortality. Survival analysis stratified by ART status showed lower mortality among naïve compared with non-naïve patients, but there were no differences between patients receiving either efavirenz or non-efavirenz ART regimens (Additional file 1: Figure S2).
Marginal structural models
Of the 1389 patients in the prospective HIV:TB study 229(16.5%) never started ART and were therefore, only included in the marginal structural model. Of these 229 patients 92% were from EE, 4% from WE and 3% LA. Their median CD4 cell count was 149 cells/uL. 60% were IDU, 38% had MDR-TB and 65% had disseminated TB (see Additional file 1: Table S2). Overall 117(51%) died, and at 12 months after TB diagnosis 75(33%) were lost to follow up. Using the marginal structural model the odds of death (OR) for those in receipt of an efavirenz-containing ART regimen was 0.82 (95%CI: 0.66–1.02, p = 0.08, when compared to those receiving a non-efavirenz containing regimen. After truncating weights for different percentiles, such as 95%, the mortality odds remained non-significant (OR 0.85 (95%CI: 0.67–1.07, p = 0.18). These results, obtained using this model were consistent with the other statistical techniques used in this study. Furthermore exploration, in an analysis restricted to patients starting efavirenz compared with those starting a ritonavir-boosted protease inhibitor regimen only, showed that the odds of death for those who started an efavirenz-containing ART regimen was statistically better (OR 0.76, 95%CI: 0.60–0.95, p = 0.01).
Sensitivity analysis for mortality among ART-naïve patients
This analysis included 518 ART-naïve patients: 420 (81%) receiving efavirenz-based ART and 98 (19%) non-efavirenz containing ART. Adjusted survival curves are shown in Additional file 1: Figure S3 and the Cox model results are shown in Additional file 1: Table S1. In the marginal structural model, odds of death were better in those receiving efavirenz-based ART: OR 0.55 (95%CI: 0.36–0.85, p < 0.01). Truncating at other percentiles showed similar results. Additional file 1: Figure S4 and Table S3 show the results for ART-non naïve patients.
Virological suppression
Among 453 of the original 965 patients who still were alive and in care at 12 months, and who had viral load information available, 352 (77%) patients had undetectable plasma HIV viral loads (less than 400 copies/ml). The proportion of patients with virological suppression was less frequent in EE, regardless of the ART regimen, compared to the other regions. Distribution by ART regimen and treatment experience is shown in Table 2. In an adjusted Cox model, the hazard ratio (HR) for virological suppression was 0.97 (95%CI: 0.76–1.22: p = 0.77) for efavirenz, compared with non-efavirenz containing ART regimens. Gender, age, route of HIV transmission, disseminated TB, rifamycin use, being ART-naïve, and having documented MDR-TB were not significantly associated with virological suppression (Table 4).
Loss to follow-up
Of the 852 patients included in this analysis, which only excluded patients who died, 179 (21%) were lost to follow up at 12 months (Table 2). In a Cox model, adjusted for gender, age, region, CD4 count at diagnosis of TB, loss to follow-up was similar among those receiving an efavirenz-containing regimen of ART, when compared with non-efavirenz containing regimens: HR 1.17 (95% CI: 0.81–1.67: p = 0.40). However, a higher risk of loss to follow-up was found in patients from EE, compared to patients from other regions (Table 5).
Discussion
In this prospective observational study, there were major differences in outcomes that were predicated by patients’ region of residence. The mortality in Eastern Europe was significantly higher than in other regions, as described previously [3, 34, 35], but this did not differ significantly according to ART regimen (either efavirenz or non-efavirenz containing ART). Although ART-naïve patients had slightly better survival in comparison to ART non-naïve patients, this was similar for patients receiving efavirenz and non-efavirenz containing ART regimens. Furthermore, the proportions of patients who became lost to follow up and who had undetectable viral loads at 12 months following diagnosis of TB did not differ according to whether efavirenz was included in the ART regimen.
The observed similar survival in patients treated with efavirenz or non-efavirenz regimens may have several explanations, including that the non-efavirenz group was heterogeneous. Although inverse probability weighting was used to balance the differences in the co-variables between groups, it is possible that not all confounders including socio-demographic factors, comorbidities and/or concomitant (opportunistic) infections, diagnostic delay, and access to anti-TB drug susceptibility tests were included. In contrast with previously described findings in this cohort, MDR-TB was not associated with mortality at 12 months, probably because those with MDR-TB were less likely to start antiretroviral therapy and were therefore not included in the current study: moreover, a resistance test was performed in only 54% of the selected population. In the main model used, no differences in mortality between regimes was observed, but in an explorative marginal structural model restricted just to patients treated with a boosted protease inhibitor or efavirenz, survival was statistically different, favoring the group receiving efavirenz-based ART. This finding may reflect poorer tolerability of protease inhibitors, greater frequency of drug-drug interactions, or previous virological failure that could additionally impact on mortality. Surprisingly, there was a high proportion of subjects receiving PI-based ART as their first regimen (57%). This group of patients were mostly in the Eastern European region (61%), and most likely the reason for initiating ART with a PI-containing regimen was because of the local availability. Moreover, only 22% of those on non-efavirenz regimens received integrase inhibitors, a majority from Western Europe. Any inferences based on outcomes with this class of ART should be interpreted with caution as the study was not designed to directly compare integrase inhibitors with efavirenz, and additionally, the main analysis may have lacked power to detect a survival benefit.
The proportion of patients who were lost to follow up was high, especially among patients from Eastern Europe, irrespective of ART regimen. This is consistent with findings from other studies in countries with a high burden of TB [36]. Higher rates of loss to follow up in some other studies may be explained by differences in patient populations, health care provision models including more assertive outreach to patients who have dropped out of care. Although frequency of patients with virological suppression at 12 month was overall acceptable (77%), the proportion of plasma HIV-RNA determinations was heterogeneous between regions. Nevertheless, if the goal is to reach the 90–90-90 target, this rate of virological suppression is still some way from being accomplished. No clinical factors associated with an undetectable HIV viral load were found in this study, however it was less likely to be achieved in Eastern Europe. In general, unfavorable outcomes such as loss to follow-up, proportion of detectable viral load and mortality were more frequent in the Eastern European region, which is consistent with previous publications from our study [2, 3]. Factors such as characteristics of the HIV population (i.e. the high proportion of IDU), education and health care barriers, less access to HIV monitoring such as HIV-RNA determinations, and the feasibility of implementing current recommendations on ART initiation in the context of an AIDS-defining event [37], might have an important role when patients are compared with other regions with limited healthcare resources, such as Latin America.
Our study has several limitations. These include the relatively short follow-up period, differences between regions in terms of routine care such as access to HIV-RNA determinations, heterogeneity of use of ART, availability of rifabutin and integrase inhibitors, and timing of ART initiation at TB diagnosis. In terms of data availability, we recognize limitations in information regarding reasons for initiating or changing specific ART regimens, which did not allow us to accurately distinguish between changes due to adverse effects or changes due to ART failure and we cannot exclude the possibility that some naïve patients might have been receiving ART – although this was not detected during our quality assurance procedures. However, this is the largest multi-regional TB:HIV cohort and the first to comprehensively compare the concomitant use of TB treatment and efavirenz and non-efavirenz containing ART regimes. Using marginal structural models, there was a slightly better outcome for patients treated with efavirenz-containing ART, although this was not consistent in all analyses and therefore not categorical. These results warrant further analyses.
Conclusions
In this multi-centre, multi-region cohort study, overall, poor outcomes were more frequent in Eastern Europe. Use of efavirenz or non-efavirenz containing ART regimens did not impact on mortality, virological suppression, or loss to follow-up at 12 months following diagnosis of TB. Considering these differences between regions irrespective of ART regimen, more, prospective studies are needed, particularly in resource-constrained regions in which access to rifabutin and integrase inhibitors is still limited.
References
WHO. Global tuberculosis report. World Health Organization, 2014.
Podlekareva DN, Mocroft A, Post FA, Riekstina V, Miro JM, Furrer H, et al. Mortality from HIV and TB coinfections is higher in Eastern Europe than in Western Europe and Argentina. AIDS. 2009;23(18):2485–95.
Podlekareva DN, Efsen AM, Schultze A, Post FA, Skrahina AM, Panteleev A, et al. Tuberculosis-related mortality in people living with HIV in Europe and Latin America: an international cohort study. Lancet HIV. 2016;3(3):e120–31.
Mukadi YD, Maher D, Harries A. Tuberculosis case fatality rates in high HIV prevalence populations in sub-Saharan Africa. AIDS. 2001;15(2):143–52.
Zhou J, Elliott J, Li PC, Lim PL, Kiertiburanakul S, Kumarasamy N, et al. Risk and prognostic significance of tuberculosis in patients from the TREAT Asia HIV observational database. BMC Infect Dis. 2009;9:46.
Gupta RK, Lucas SB, Fielding KL, Lawn SD. Prevalence of tuberculosis in post-mortem studies of HIV-infected adults and children in resource-limited settings: a systematic review and meta-analysis. AIDS. 2015;29(15):1987–2002.
Balcha TT, Skogmar S, Sturegard E, Bjorkman P, Winqvist N. Outcome of tuberculosis treatment in HIV-positive adults diagnosed through active versus passive case-finding. Glob Health Action. 2015;8:27048.
Manosuthi W, Wiboonchutikul S, Sungkanuparph S. Integrated therapy for HIV and tuberculosis. AIDS Res Ther. 2016;13:22.
Havlir DV, Kendall MA, Ive P, Kumwenda J, Swindells S, Qasba SS, et al. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med. 2011;365(16):1482–91.
Blanc FX, Sok T, Laureillard D, Borand L, Rekacewicz C, Nerrienet E, et al. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med. 2011;365(16):1471–81.
Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray AL, et al. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med. 2011;365(16):1492–501.
Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362(8):697–706.
Schwander S, Rusch-Gerdes S, Mateega A, Lutalo T, Tugume S, Kityo C, et al. A pilot study of antituberculosis combinations comparing rifabutin with rifampicin in the treatment of HIV-1 associated tuberculosis. A single-blind randomized evaluation in Ugandan patients with HIV-1 infection and pulmonary tuberculosis. Tuber Lung Dis. 1995;76(3):210–8.
Chien JY, Chien ST, Huang SY, Yu CJ. Safety of rifabutin replacing rifampicin in the treatment of tuberculosis: a single-Centre retrospective cohort study. J Antimicrob Chemother. 2014;69(3):790–6.
Rawson TM, Brima N, Almajid F, Pozniak AL, Janmohamed A, Mandalia S, et al. Outcomes from treating tuberculosis with rifampicin or rifabutin in HIV-infected persons also receiving antiretroviral therapy. J Acquir Immune Defic Syndr. 2015;68(5):e84–7.
WHO. Priority research questions for tuberculosis/human immunodeficiency virus (TB/HIV) in HIV-prevalent and resource-limited settings. Geneva: World Health Organization; 2010.
WHO. Tuberculosis care with TB-HIV co-management : integrated Management of Adolescent and Adult Illness (IMAI). 2007.
WHO. Guidelines for national programmes and other stakeholders. 2012.
Migliori GB, Zellweger JP, Abubakar I, Ibraim E, Caminero JA, De Vries G, et al. European union standards for tuberculosis care. Eur Respir J. 2012;39(4):807–19.
National Association Phthisiology. Clinical recommendations diagnosis and treatment of tuberculosis respiratory organs in adults, (homepage on the Internet). 2008 (cited 2017 Jan 17). Available from: Russia, Web site: http://itpcru.org/wpcontent/uploads/2014/09/Klinicheskie-rekomendatsii-TB-RF-2014.pdf.
Departamentul Central De Management Al Pnct. GUIDE implementation methodology of the National Tuberculosis Control, (homepage on the Internet). 2007 (cited 2017 Jan 17). Available from: Rumania, Web site: http://www.srp.ro/ghiduri/Ghid%20metodologic%20TBC%202007-2011.doc.
Agenda of the Minister of Health. Appendix to the Bulletin of the National Center for. AIDS. (homepage on the Internet). 2011 (cited 2017 Jan 17). Available from: Poland, Web site: http://www.aids.gov.pl/pobierz/1642/.
Avila D, Althoff KN, Mugglin C, Wools-Kaloustian K, Koller M, Dabis F, et al. Immunodeficiency at the start of combination antiretroviral therapy in low-, middle-, and high-income countries. J Acquir Immune Defic Syndr. 2014;65(1):e8–16.
Crabtree-Ramirez B, Caro-Vega Y, Shepherd BE, Wehbe F, Cesar C, Cortes C, et al. Cross-sectional analysis of late HAART initiation in Latin America and the Caribbean: late testers and late presenters. PLoS One. 2011;6(5):e20272.
Mocroft A, Lundgren J, Antinori A, Monforte A, Brannstrom J, Bonnet F, et al. Late presentation for HIV care across Europe: update from the collaboration of observational HIV epidemiological research Europe (COHERE) study, 2010 to 2013. Euro Surveill. 2015;20(47) https://doi.org/10.2807/1560-7917.ES.2015.20.47.30070.
Boulle A, Van Cutsem G, Cohen K, Hilderbrand K, Mathee S, Abrahams M, et al. Outcomes of nevirapine- and efavirenz-based antiretroviral therapy when coadministered with rifampicin-based antitubercular therapy. JAMA. 2008;300(5):530–9.
Murphy RA, Marconi VC, Gandhi RT, Kuritzkes DR, Sunpath H. Coadministration of lopinavir/ritonavir and rifampicin in HIV and tuberculosis co-infected adults in South Africa. PLoS One. 2012;7(9):e44793.
Maartens G, Decloedt E, Cohen K. Effectiveness and safety of antiretrovirals with rifampicin: crucial issues for high-burden countries. Antivir Ther. 2009;14(8):1039–43.
Minchella PA, Armitage AE, Darboe B, Jallow MW, Drakesmith H, Jaye A, et al. Elevated hepcidin at HIV diagnosis is associated with incident tuberculosis in a retrospective cohort study. Int J Tuberc Lung Dis. 2014;18(11):1337–9.
Grinsztejn B, De Castro N, Arnold V, Veloso VG, Morgado M, Pilotto JH, et al. Raltegravir for the treatment of patients co-infected with HIV and tuberculosis (ANRS 12 180 reflate TB): a multicentre, phase 2, non-comparative, open-label, randomised trial. Lancet Infect Dis. 2014;14(6):459–67.
CDC. Managing Drug Interactions in the Treatment of HIV-Related Tuberculosis Available from: www.cdc.gov/tb/publications/guidelines/tb_hiv_drugs/recommendations03.htm.
Howe CJ, Cole SR, Westreich DJ, Greenland S, Napravnik S, Eron JJ Jr. Splines for trend analysis and continuous confounder control. Epidemiology. 2011;22(6):874–5.
Cain LE, Robins JM, Lanoy E, Logan R, Costagliola D, Hernan MA. When to start treatment? A systematic approach to the comparison of dynamic regimes using observational data. Int J Biostat. 2010;6(2):Article 18.
TB: HIV Study writing Group. One-year mortality of HIV-positive patients treated for rifampicin- and isoniazidsusceptible tuberculosis in Eastern Europe, Western Europe, and Latin America. AIDS. 2017;31(3):375–84.
Efsen AM, Schultze A, Post FA, Panteleev A, Furrer H, Miller RF, et al. Major challenges in clinical management of TB/HIV Coinfected patients in Eastern Europe compared with Western Europe and Latin America. PLoS One. 2015;10(12):e0145380.
Bassett IV, Chetty S, Wang B, Mazibuko M, Giddy J, Lu Z, et al. Loss to follow-up and mortality among HIV-infected people co-infected with TB at ART initiation in Durban, South Africa. J Acquir Immune Defic Syndr. 2012;59(1):25–30.
Crabtree-Ramirez B, Caro-Vega Y, Shepherd BE, Grinsztejn B, Wolff M, Cortes CP, et al. Time to HAART initiation after diagnosis and treatment of opportunistic infections in patients with AIDS in Latin America. PLoS One. 2016;11(6):e0153921.
Acknowledgements
We thank the patients who participated in the study and the staff involved at the participating hospitals in the TB:HIV study group.
TB:HIV Study group.
Eastern Europe: Belarus: Belarusian State Medical University, Department of Infectious Disease: I. Karpov (PI), A. Vassilenko; Republican Research and Practical Centre for Pulmonology and TB (Minsk): A. Skrahina (PI), D. Klimuk, A. Skrahin, O. Kondratenko and A. Zalutskaya; Gomel State Medical University (Gomel): V. Bondarenko (PI), V. Mitsura, E. Kozorez, O. Tumash; Gomel Region Centre for Hygiene: O. Suetnov (PI) and D. Paduto. Estonia: East Viru Central Hospital (Kohtla-Jarve): V. Iljina (PI) and T. Kummik. Georgia: Infectious Diseases, AIDS and Clinical Immunology Research Center (Tiblisi): N. Bolokadze (PI), K. Mshvidobadze and N. Lanchava; National Center for Tuberculosis and Lung Diseases of Georgia (Tibilisi): L. Goginashvili, L. Mikiashvili and N. Bablishvili. Latvia: Infectology Centre of Latvia (Riga): B. Rozentale (PI), I. Zeltina and I. Janushkevich. Lithuania: Centre for Communicable Diseases and AIDS (Vilnius): I. Caplinskiene (PI), S. Caplinskas, Z. Kancauskiene. Poland: Wojewodski Szpital Zakanzy/Medical University of.
Warsaw (Warszawa): R. Podlasin (PI), A. Wiercinska-Drapalo (PI), M. Thompson and J. Kozlowska; Wojewodski Szpital Specjalistyczny/Medical University Teaching Hospital (Bialystok): A. Grezesczuk (PI); Jozef Strus Multidisciplinary City Hospital (Poznan): M. Bura (PI); Wroclaw University School of Medicine (Wroclaw): B. Knysz (PI) and M. Inglot; Jagiellonian University Medical College (Krakow): A. Garlicki (PI) and J. Loster. Romania: Dr. Victor Babes Hospital (Bucharest): D. Duiculescu († PI) and S. Tetradov. Russia: Botkin Hospital of Infectious Diseases (St. Petersburg): A. Rakhmanova († PI), O. Panteleeva, A. Yakovlev, A. Kozlov, A. Tyukalova and Y. Vlasova; City TB Hospital No. 2 (St. Petersburg): A. Panteleev (PI); Center for Prevention and Control of AIDS (Veliky, Novgorod): T. Trofimov (PI); Medical University Povoljskiy Federal Region. Ukraine: Crimean Republican AIDS Centre (Simferopol): G. Kyselyova (PI).
Western Europe: Belgium: CHU Saint-Pierre (Brussels): M.C. Payen (PI), K. Kabeya and C. Necsoi. Denmark: Rigshospitalet (Copenhagen): N. Obel (PI); Hvidovre University Hospital: K. Thorsteinsson. France: Aquitaine Cohort. Cohort administration: F. Dabis (PI) and E. Pernot. Participating Centers and Physicians: Bordeaux University Hospital: P. Morlat; Arcachon Hospital: A. Dupont; Dax Hospital: Y. Gerard; Bayonne Hospital: F. Bonnal; Libourne Hospital: J. Ceccaldi; Mont-de-Marsan Hospital: S. De Witte; Pau Hospital: E. Monlun; Périgueux Hospital: P. Lataste; Villeneuve-sur-Lot Hospital: I. Chossat. Switzerland: Swiss HIV Cohort Study (SHCS, www.shcs.ch): Cohort administration: M. Sagette and M. Rickenbach. Participating Centers and Physicians: University Hospital Basel: L. Elzi and M. Battegay; University Hospital Bern: H. Furrer (PI); Hopital Cantonal Universitaire, Geneve: D. Sculier and A. Calmy; Centre Hospitalaire Universitaire Vaudois, Lausanne: M. Cavassini; Hospital of Lugano: A. Bruno and E. Bernasconi; Cantonal Hospital St. Gallen: M. Hoffmann and P. Vernazza; University Hospital Zurich: J. Fehr and R. Weber. This study has been co-financed within the framework of the Swiss HIV Cohort Study, supported by the Swiss National Science Foundation (grant #148522) and by SHCS project 666. The data are gathered by the Five Swiss University Hospitals, two Cantonal Hospitals, 15 affiliated hospitals and 36 private physicians). The members of the Swiss HIV Cohort Study are: V. Aubert, M. Battegay, E. Bernasconi, J. Böni, H.C. Bucher, C. Burton-Jeangros, A. Calmy, M. Cavassini, G. Dollenmaier, M. Egger, L. Elzi, F. Fehr, J. Fellay, H. Furrer (Chairman of the Clinical and Laboratory Committee), C.A. Fux, M. Gorgievski, H. Günthard (President of the SHCS), D. Haerry (deputy of “Positive Council”), B. Hasse, H.H. Hirsch, M. Hoffmann, I. Hösli, C. Kahlert, L. Kaiser, O. Keiser, T. Limkait, R. Kouyos, H. Kovari, B. Ledergerber, G. Martinetti, B. Martinez de Tejada, K. Metzner, N. Müller, D. Nadal, D. Nicca, G. Pantaleo, A. Rauch (Chairman of the Scientific Board), S. Regenass, M. Rickenbach (Head of Data Center), C. Rudin (Chairman of the Mother & Child Sub-study), F. Schöni-Affolter, P. Schmid, J. Schüpbach, R. Speck, P. Tarr, A. Telenti, A. Trkola, P. Vernazza, R. Weber, S. Yerly. United Kingdom: Mortimer Market Centre (London): R.F. Miller (PI) and N. Vora; St. Mary’s Hospital (London): G. Cooke (PI) and S. Mullaney; North Manchester General Hospital: E. Wilkins (PI) and V. George; Sheffield Teaching Hospitals: P. Collini (PI) and D. Dockrell; King’s College Hospital (London): F.A. Post (PI), L. Campbell, R. Brum, E. Mabonga and P. Saigal. Queen Elizabeth Hospital: S. Kegg (PI); North Middlesex University Hospital: J. Ainsworth (PI) and A. Waters. Leicester Royal Infirmary: J. Dhar (PI) and L. Mashonganyika. Southern Europe: Italy: IRCCS - Ospedale L. Spallanzani (Rome): E. Girardi (PI), A Rianda, V. Galati, C. Pinnetti and C. Tommasi; AO San Gerardo (Monza): G. Lapadula (PI); IRCCS AOU San Martino – IST di Genoa (Genova): A. Di Biagio (PI) and A. Parisini; Clinic of Infectious Diseases, University of Bari (Bari): S. Carbonara (PI), G. Angarano and M. Purgatorio; University of Brescia Spedali Civili: A. Matteelli (PI) and A. Apostoli. Spain: Barcelona Cohort funded by the Spanish HIV/AIDS Research Network: Hospital Clinic of Barcelona: J.M. Miro (PI), C. Manzardo, C. Ligero and J. Gonzalez; Hospital del Mar: F. Sanchez, H. Knobel, M. Salvadó and J.L. Lopez-Colomes; Mutua de Terrassa: X. Martínez-Lacasa and E. Cuchí; Hospital Universitari Vall d’Hebrón: V. Falcó, A. Curran, M.T. Tortola, I. Ocaña and R. Vidal; Hospital Universitari de la Santa Creu i Sant Pau: M.A. Sambeat, V. Pomar and P. Coll; Hospital Universitari de Bellvitge: D. Pozamczer, M. Saumoy and F. Alcaide; Agencia de Salud Pública de Barcelona: J. Caylà, A. Moreno, J.P. Millet, A. Orcau, L. Fina, L. del Baño, L.L. Roldan. Hospital Universitario Donostia (San Sebastian): JA. Iribarren (PI) and M. Ibarguren; Hospital Universitario Ramon y Cajal (Madrid): S. Moreno (PI) and A. González; Hospital Universitario ‘Gregorio Maranon’ (Madrid): P. Miralles (PI) and T. Aldámiz-Echevarría.
Latin America: Argentina: The CICAL Cohort: Cohorte administration: M. Losso (PI), J. Toibaro and L. Gambardella. Participating Centers and Physicians: Argentina: Hospital J. M. Ramos Mejía (Buenos Aires): J. Toibaro and L. Moreno Macias; Hospital Paroissien (BA): E. W arley (PI) and S. Tavella; Hospital Piñero (BA): O. Garcia Messina and O. Gear; Hospital Nacional Profesor Alejandro Posadas: H. Laplume; Hospital Rawson (Cordoba): C. Marson (PI); Hospital San Juan de Dios (La Plata): J. Contarelia and M. Michaan; Hospital General de Agudos Donación F. Santojani: P. Scapellato and D. D Alessandro; Hospital Francisco Javier Muñiz (BA): B. Bartoletti and D. Palmero; Hospital Jujuy: C. Elias. Chile: Fundación Arriaran (Santiago): C. Cortes. México: INNCMSZ (México DF): B. Crabtree (PI); Hospital Civil de Guadalajara (Guadalajara): J. Andrade (PI); Hospital General Regional de Leon-CAPASITS: J.L. Mosqueda Gomez.
† Deceased.
TB:HIV Steering Committee: H. Furrer, E. Girardi, M. Bruyand, J.A. Caylá, M. Losso, J.D. Lundgren, A. Panteleev (co-chair), R.F. Miller, J.M. Miro, Å.B. Andersen, S. Tetradov, F.A. Post (co-chair), A. Skrahin and J. Toibaro.
Statistical centre: A. Schultze, L. Shepherd, A. Mocroft.
Coordinating centre: A.M.W. Efsen, M. Mansfeld, B. Aagaard, B.R. Nielsen, A H. Fisher, R.S. Brandt, D. Raben, D.N. Podlekareva, O. Kirk.
Funding
This study was funded by the European Union’s Seventh Framework Programme for research (FP7/2007–2013), technological development and demonstration under EuroCoord grant agreement number 260694, the Danish Council for Independent Research, the Danish National Research Foundation (grant 126) and the Research Council, Rigshospitalet (Copenhagen, Denmark). TB:HIV study data were pooled with the EuroCoord network.
This work was partially supported by the NIH-funded Caribbean, Central and South America network for HIV epidemiology (CCASAnet), a member cohort of the International Epidemiologic Databases to Evaluate AIDS (leDEA) (U01AI069923). This award is funded by the following institutes: Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD), National Cancer Institute (NCI), National Institute of Allergy And Infectious Diseases (NIAID), National Institute of Mental Health (NIMH), and the Office of The Director, National Institutes of Health (OD).
Availability of data and materials
The database contains person-sensitive information and is therefore not publicly available. The TB:HIV Steering Committee encourages the submission of concepts for research proposals. Concepts can be submitted for review using an online research concept, please see our website (https://www.chip.dk/Studies/TBHIV/Submit-research-concept). The concept will be evaluated by the Steering Committee for scientific relevance, relevance to the TB:HIV study, design, feasibility and overlap with already approved projects.
All proposers will receive feedback and revision of the concept may be requested. If approved, a writing group will be established consisting of proposers, members of the Steering Committee and staff at the coordinating center and the statistical center. The TB:HIV Study can be accessed at www.chip.dk/TBHIV, where all relevant documents are available (study protocol, CRF’s, newsletters, presentations and publications etc.). All relevant staff involved in the study and their contact information is also available at this website. For submission of research proposal, please contact Anne Marie Werlinrud Efsen (anne.marie.werlinrud.efsen@regionh.dk) and Ole Kirk (ole.kirk@regionh.dk).
Author information
Authors and Affiliations
Contributions
YCV, AS1, and AMWE, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: BCR, JSM, ML and YCV. Acquisition of data: AMWE, AP, AS1, JMM, EG, DP, JDL, JT, JAV, ST, JF, JC, ML, RM, OK, and BCR. Analysis and interpretation of data: BCR, YCV, AS1, AMWE, FP, AM and OK. Drafting of the manuscript: YCV and BCR. Critical revision of the manuscript for important intellectual content: YCV, AS1, AMWE, FP, ML, RM, OK, BCR, AP, AS2, JMM, EG, DP, JDL, JT, JAV, ST, JF, JC and AM. Statistical analysis: YCV, AS1 and AM. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The HIV/TB project was an observational study and patients were not exposed to any experimental interventions nor did the study intervene with the clinical management of the patient. The study only collected information from patient records and if necessary, patient interview. The study was conducted according to the current ethical standards including the WMA Declaration of Helsinki and was submitted to the appropriate regulatory authorities including ethical committees in the participating countries, as requested by local regulations. When informed consent was required by the local and/or national Ethics Committees, this was obtained prior to the initiation of any study related data being obtained. The consent form was approved by the IEC/IRB of each participating centre.
In specific, IEC/IRB were obtained from the following commitees:
Le Comité d’Ethique du C.H.U Saint-Pierre, Le Numéra registre AK/12–03-28/4128, Comité.
Ético Cientifico del servicio de Salud Metropolitano Centra, Chil, certificado 452/11, De.
Videnskabsetiske Komiteer i Region Hovedstaden Journal nr.: H-3-2011-095, United Kingdom national ethics approval Reference nr. 11/LO/0713 & R&D Reference nr. CSP 75430 for UK sites: Mortimor Market Centre, London, Imperial College Healthcare, London, St. Marys Hospital, London, North Manchester General Hospital, Manchester, Sheffield Teaching Hospitals, Sheffield, King’s College Hospital, London, North Middlesex University Hospital, London, Queen Elisabeth Hospital, London R&D ref. nr. SLHT/2011/UCSM/HIV/88, Leicester Royal Infirmary, Leicester. El Comité de Etica en Investigación en Salud, Hospital Paroissien Argentina 20/12/2911, Comité de Etica Iniciativa y Reflextion Bioetica Hospital Pineiro & Hospital Ramos Mejia Argentina 12/12/12, Comité de Bioética Reg. nr. 11,743/11 Hospital Nacional Profesor Alejandro Posdas Argentina, Comité Institucional de Ètica de la Investigacion en Salud del Nino u del Adulto Cordoba 16/12/2012 Hospital Rawson, Comité de Etica La Plata 12/11/12 Hospital Sa Juan de Dios Argentina, Comité de Ética en Investigacion Buenos Aires 18/11/12 Hospital Santojani Argentina & 11/05/2012 Hospital Fracisco Javier Muniz Argentina, Docencia e Investigación 15/06/12 Jujuy Argentina, Institutional Review Board Tbilisi Ref. nr. 12–007, Registro delle Sperimentazioni del Comitato Etico Rone nr. 24/2011 Spallanzani, Comitato Etico San Gerardo, Monza 26/05/2011, Comitato Etico Aziendale A.O.U. San Martino Genoa nr. 295 08/04/2011, Comitato Etico Indipendente Locale Policlinio Consorziale Bari nr. 624 28/09/2011, Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán Ref. nr 437, Comité de Ética del Hospital General Regional de León 10/10/2011, Secretario del Comité de Ensenanza, Investigacion y Ética Hospital Civil de Guadalajara Invest. Nr. 038/12, Comitato Etico Spedali Civili Brescia 07/06/2011 nr.36; Direcció General de Regularcio Barcelona CY-ANT-2011-01, Hospital Universitario Ramón y Cajal Comité Ético de Investigación Clinica 12/07/2011, Comite Etico de Investigation Clinica de Euscadi Vitoria 02/02/2012, Comité Ético de Investigación Clínica Hospital General Universitario Gregorio Maranon Madrid SAS/3470/2009, Kantonale Ethik-Kommission Zürich EK-793, Ethical Committee of Rep. Res. And Practical Centre for Pulmonology Minsk 11/02/2011, Ethical Committee of Gomel State Medical University & Gomel Regional Centre for Hygiene, Ethical Committee for Botkin Hospital & City TB Hospital ref. nr.23, Ethical Committee for Novgorod Centre for AIDS Prevention and Control 11/10/2011, Ethican Committee of Samara State Medical University 10/10/2012, Komisja Bioetycznego Uniwersytetu Medycznego w Bialymstoku Poland R-I-00/85c/2012 –R-I-002/85/2011, Tallinn Medical Research Ethics Committee Appr. Nr. 2555, RSU Etikas Komitejas Lemums Riga Nr. E-9(2), Vilniaus Regioninis Biomedicininiu Tyrimu Etikos Komitetas Nr. 158,200–07–363-90, Dr. Victor Babes Hospital Institutional Ethics Committee nr. 3889, Ethics Committee Crimean Republican AIDS Centre Nr. 462–13–08-12.
The data storage and handling was protected in accordance with and approved by the Danish Data Protection Agency (Datatilsynet) under the Act on Processing of Personal Data (Act No. 429 of 31 May 2000) and European Commission Directive 95/46/EC. http://www.chip.dk/Portals/0/files/Study%20documents/HIV_TB_project_protocol_2011.pdf
Competing interests
YCV, AS1, AMWE, AP, AS2, DNP, JDL, ST, ML, JT, JF, JC, JAV have no competing interests to declare.
FAP, received honoraria and/or research funding from Abbvie, Gilead, ViiV, BMS, MSD and Janssen.
JMM received a personal intensification research grant (number INT15/00168) during 2016 from the Instituto de Salud Carlos III, Ministerio de Economía y Competitividad, Madrid, Spain, and a personal 80:20 research grant from the Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Barcelona, Spain, during 2017–19.
RFM has received honoraria from Gilead, Janssen, Merck, and ViiV for giving non-promotional lectures on clinical aspects of HIV; is a member of the British HIV Association Tuberculosis/HIV Guidelines Committee; and is a panel member for the Guidelines for the Prevention and Treatment of Opportunistic Infections in HIV-Infected Adults and Adolescents (National Institutes of Health, Centers for Disease Control and Prevention, HIV Medicine Association of the Infectious Diseases Society of America).
JSM has received lecture fees, sponsorship, honoraria from Gilead, Stendhal, Abbvie, ViiV, Janssen and MSD.
EG, Consultancy for Otsuka Novel Products, Speaker’s fee from Janssen, Gilead sciences, Angelini, Research Grant from Gilead sciences, Travel Grant from Janssen.
AM, has have received lecture fees, sponsorship, honoraria or consultancy fees from ViiV, Pfizer, BMS, BI, Merck and Wragge LLC.
OK had prior board membership at ViiV Healthcare, Gilead Sciences and Merck, received payment for lectures and/or for development of educational presentations from Abbott, Gilead Sciences, Tibotec and Quagen, and had travel/accommodations/meeting expenses paid by Abbott, BMS, Gilead Sciences, Merck and ViiV Healthcare – all outside the submitted work.
BCR, has received lecture fees, sponsorship, honoraria from Gilead, Stendhal, Abbvie, Janssen and MSD.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Supplementary material response to ART in HIV-TB. Figure S1. Probability of death in efavirenz and non-efavirenz containing ART groups by inverse probability weighting method. Figure S2. Cox adjusted survival by ART regimen during the first year stratified by naïve status. Figure S3. Adjusted probability of death in the efavirenz group compared to the non-efavirenz group for naïve patients only. Table S1. Risk factors for death in naïve patients. Figure S4. Adjusted probability of death in the efavirenz group compared to the non-efavirenz group for non-naïve patients only. Table S2. Demographic and clinical characteristics of patients who never started ART at the time of starting TB treatment. Table S3. Risk factors for death in non-naïve patients. (DOCX 1262 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Caro-Vega, Y., Schultze, A., W. Efsen, A.M. et al. Differences in response to antiretroviral therapy in HIV-positive patients being treated for tuberculosis in Eastern Europe, Western Europe and Latin America. BMC Infect Dis 18, 191 (2018). https://doi.org/10.1186/s12879-018-3077-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12879-018-3077-x