Abstract
Background
Interdisciplinary geriatric consultation teams (IGCT) are regularly requested to provide comprehensive geriatric assessments in older inpatients. Our primary aim was to evaluate whether medication reviews increased the number of IGCT-provided drug-related recommendations. Secondary aims were to reduce the number of potentially inappropriate medications (PIMs), and to identify the acceptance rate of and determinants for the number of recommendations.
Methods
A before-after study was performed in older inpatients not admitted to acute geriatric wards. The before cohort received usual care (UC); the after cohort was subjected to the intervention (I), consisting of a systematic medication review, based on but not limited to the RASP (Rationalization of Home Medication by an Adjusted STOPP in Older Patients) list. The primary outcome measure was the number of IGCT-provided drug-related recommendations. Age, sex, Charlson Comorbidity Index, creatinine clearance and serum creatinine were ascertained upon enrolment. Following variables were determined on admission and at discharge: number of drugs and number as well as type of RASP-identified PIMs. Acceptance by ward-based physicians was also determined. Poisson regression was performed to identify determinants for the primary outcome measure.
Results
Fifty-nine participants were enrolled (nUC = 29; nI = 30). The intervention increased the number of drug-related recommendations from a median of 0 (IQR: 0–1) to 8 (IQR: 6.75–10) (p < 0.001). The median number of accepted recommendations differed significantly as well (UC vs. I: 0.0 (0.0–0.5) vs. 3.0 (0.0–5.3); p < 0.001). In the intervention cohort, patients were discharged with fewer drugs compared to admission (UC vs. I: 108.5%, IQR: 100.0–135.8% vs. 92%, IQR: 80.5–103.5%; p = 0.002). More RASP PIMs were discontinued in the intervention cohort, with a mean difference of 1.49 RASP PIMs (95% confidence interval (CI): 0.70, 2.23; p < 0.001). Regression analysis identified two determinants: allocation to the intervention cohort with an incidence rate ratio (IRR) of 14.1 (95% CI: 8.30, 23.8) and the number of preadmission drugs with an IRR of 1.06 (95% CI: 1.03, 1.09).
Conclusions
A structured medication review as part of usual IGCT care may contribute to an increased detection of drug-related problems and help to further reduce polypharmacy in older inpatients, not admitted to acute geriatric care wards.
Trial registration
NCT02165618, retrospectively registered June 17, 2014.
Similar content being viewed by others
Background
Inappropriate drug use remains common in older persons and has been associated with a lower quality of life, increased health-care utilization and an increased readmission risk [1]. Causes are many and frequently complex, yet have been shown to be at least partially amenable to change [1,2,3,4,5,6,7,8].
Multiple comprehensive interventions have already been demonstrated to improve the quality of prescribing and to reduce the number of potentially inappropriate medications (PIMs). Most interventions have been limited to the inpatient setting, which might partially be explained by the provision of increased monitoring during hospital stay and also by higher baseline risk of the hospitalized patient population for adverse (drug) events, resulting in a relatively lower needed sample size for interventional studies [9]. In several trials, such interventions relied on the addition of ward-based hospital pharmacists to the multidisciplinary care team [4]. Hospital pharmacists provided several services, such as medication reconciliation on admission, supporting the implementation and adjustment of clinical decision support systems (CDSS), patient education and medication review [2, 5, 8, 10]. Pharmacist-led interventions affect clinical outcome; a decrease in the number of unplanned drug-related readmissions and emergency department visits has been observed, as concluded in recent meta-analyses [1, 4]. More data are still needed however to corroborate previous findings on the perceived benefit of medication review in older adults and also to further confirm the perceived benefits in specific subgroups [1, 4, 11, 12].
To ascertain the appropriateness of drug therapies in older adults, several screening tools have been made available [13]. We have developed the RASP (Rationalization of Home Medication by an Adjusted STOPP in Older Patients) list, which was subsequently investigated as part of a pharmacist-led medication review in a controlled trial which took place on several acute geriatric wards in a large teaching hospital [2, 13]. This approach was shown to improve the quality of prescribing without increasing harm in a very old, predominantly octogenarian, inpatient population [2]. The intervention was however restricted to patients admitted to dedicated acute geriatric care units, limiting the benefits to a select subgroup of older adults. In many hospitals the majority of octogenarians are admitted to non-geriatric wards rather than to acute geriatric care units.
Previous reports have shown that a comprehensive geriatric assessment (CGA) by the interdisciplinary geriatric consultation team (IGCT) improved care in selected inpatient populations [14,15,16]. Considering the manner in which the CGA is offered, it is still not completely elucidated whether to opt for a fully decentralized assessment of geriatric patients, to develop a co-management model or to transfer and admit high risk older patients to dedicated acute geriatric wards [17,18,19]. It has furthermore not been established whether IGCT involvement might lead to an improvement in drug use in older patients. Dalleur et al. performed a randomized controlled trial to investigate this subject. The authors concluded that the use of the STOPP criteria by trained geriatricians, as members of the IGCT, led to a doubling of the reduction of PIMs in older inpatients admitted to non-geriatric medical units in a Belgian hospital [20]. The decentral IGCT team composition and provided care have however been shown to be highly heterogeneous and results by Dalleur et al. can hence not be readily extrapolated [20, 21].
As of 2014, Belgian law has provided a fixed budget for a minimum of two full time equivalents (mostly nursing staff), excluding fees for geriatricians, who are to be financed through other means. While improving care in older inpatients is central to the IGCT care model, the specific tasks and composition of IGCTs differ substantially across hospitals [21]. In our hospital, a medication review is not performed systematically by the IGCT. Frequently, the IGCT has rather recommended to perform additional blood tests, to screen for and treat low haematocrit or haemoglobin, to organize transfer to a rehabilitation centre or to organize professional home care [14].
The aim of this pilot study (RASP-IGCT) was to evaluate whether a medication review carried out by non-geriatricians using the RASP list and provided within the context of a CGA might result in an increase of drug-related recommendations in frail older patients, who had been evaluated by the IGCT and who were admitted to non-geriatric wards. Furthermore, this study aimed to evaluate the acceptance of the recommendations, to reduce the number of PIMs and to identify significant determinants for the number of IGCT provided drug-related recommendations.
Methods
Design and setting
The RASP-IGCT study was designed as a monocentric before-after study in older inpatients, who were not admitted to acute geriatric wards. This quasi-experimental design was used, due to the potential risk of contamination bias as the same IGCT would be employed in the before (usual care) and after (intervention) cohorts. Patients enrolled in January 2014 were consecutively admitted to the usual care cohort. In February 2014, patients were consecutively admitted to the intervention cohort, in which they were subjected to a systematic medication review. Ward-based physicians were blind to the study design.
The study took place in a 2000-bed teaching hospital, the University Hospitals of Leuven, Belgium. The study was approved by the local Ethics Committee. Patients were considered for inclusion if written informed consent was provided by the patients, or by their relatives in case of them being unable to provide consent (ClinicalTrials.gov identifier NCT02165618).
Study participants
Initial assessment of the patient’s geriatric profile was performed by ward-based nurses as part of the patient assessment upon admission. The Flemish version of the six-item Triage Risk Screening Tool was used to ascertain the geriatric risk profile. A minimum score of 2 and active agreement of the treating non-geriatrician physician was needed to subsequently alert the IGCT [22].
Dutch-speaking patients, for whom an IGCT consult was requested, were eligible for inclusion if the following inclusion criteria were met: informed consent, age of 70 years or older, admission to a non-geriatric nursing ward and an enquiry for consultation from the geriatric consultation team. Patients were excluded from study participation if any of the following was applicable: no drug therapies upon admission to the hospital, presence of a terminal illness, transferal from another hospital or an acute geriatric ward, and an intensive care unit admission during hospital stay.
Baseline characteristics
Patient characteristics and baseline variables were collected upon enrolment. Following data were gathered from the admission files: age, sex, weight, number of preadmission drugs, Charlson Comorbidity Index (CCI), serum creatinine concentration (mg/dl) and estimated creatinine clearance and glomerular filtration rate according to the Cockcroft-Gault and CKD-EPI equations, respectively [23].
Furthermore, the best possible preadmission medication list was collected for all patients in a standardized manner [2, 24]. When patients were not able to adequately respond to the questions, or when their cognitive state made the answers unreliable, their family, caretakers, community pharmacist or general practitioner were contacted to verify the correctness of the medication list. If the exact medication list remained unclear, the information obtained by the general practitioner was considered to be the correct one. The RASP list was subsequently applied to the preadmission medication list; potentially inappropriate medications (PIMs) identified by the RASP list were defined as RASP PIMs. Number and identity of RASP PIMs on admission were then determined.
Usual care
Usual care was the care provided in the pre-implementation cohort by the IGCT. The IGCT performed a CGA and provided recommendations to the ward-based physician in order to improve geriatric care. A structured medication review was not applied systematically.
The team consisted of two geriatricians (JF and KF), three nurses, a social worker, two occupational therapists, and a physiotherapist. Direct patient contact was mainly carried out by the nurses. One geriatrician was present during the daily meetings, that took place on weekdays and during which all active cases were discussed. All recommendations were then added to the patient’s file.
Intervention
The study was performed by a postgraduate pharmacist (JH) and an final year undergraduate physician (LD). Both investigators were trained in the use of the RASP list and were subsequently monitored by a senior clinical pharmacist (LVDL). The reproducibility, by which both JH and LD applied the RASP, was determined by calculating Cohen’s kappa on a set of 20 anonymized sample patient records (Cohen’s kappa: < 0.00: no agreement, 0.00–0.20: slight agreement, 0.21–0.40: fair agreement, 0.41–0.60: moderate, 0.61–0.80: favourable, 0.81–1.00: almost perfect agreement) [25]. We aimed to limit the time for medication review, which included patient screening and actual enrolment, to 30 min per patient.
The investigators performed a systematic medication review, taking into account both the preadmission medication list and the prescriptions that were present in the patient’s electronic prescription file at the moment of IGCT assessment. The medication review was performed once and within 24 h after the IGCT request. Investigators used the RASP list as basis for the medication review, but were not limited to this screening tool and were allowed on a case by case basis to deviate and base their recommendations on other sources or approaches such as the Garfinkel method or the tool described by Scott et al., with a strong emphasis on identifying an indication for each drug therapy [6, 7]. Investigators were instructed beforehand by a senior clinical pharmacist, who also provided clinical supervision thereafter.
Investigators reported the drug-related recommendations, both RASP-based and non-RASP-based, directly to the IGCT during the daily meetings. Drug-related recommendations were then adopted in the IGCT notes, which were shared in the electronic patient file with other health-care professionals.
Outcome measures
The primary outcome measure was defined as the number of medication-related recommendations provided by the IGCT team. All IGCT recommendations were assessed one by one. Drug-related recommendations were subsequently identified and investigators verified whether they corresponded to any of the RASP criteria (i.e. RASP-based recommendations).
Following variables were collected as well: the identity and the number of RASP identified PIMs at discharge; the number of other medication-related (i.e. non-RASP based) recommendations; the number of drugs at discharge; the number of discontinued RASP PIMS at discharge. Furthermore, the ratio of the number of drugs at discharge to the number of drugs on admission was also calculated.
Acceptance of drug-related recommendations was defined as the agreement and subsequent change of prescription by the treating physician within 72 h after having been communicated to the physician by the IGCT. It was moreover documented whether IGCT recommendations had been adopted into the discharge letter.
Statistical analysis
Normality of continuous variables was ascertained by performing the Kolmogorov-Smirnov test and by manual, visual inspection of the histograms. Parametric variables were characterized by mean (standard deviation, SD) and non-parametric data by median (interquartile range, IQR = Q1, Q3). Proportions and counts were represented as n (%). Categorical variables, parametric data and non-parametric data were compared using Fisher Exact test, Student’s t test and the Mann-Whitney U test respectively.
A Poisson loglinear regression analysis was performed to identify determinants for the primary outcome measure of IGCT provided drug-related measures. First, an unadjusted model was developed with the intervention vs. usual care as the sole variable (model A). Then, a backward stepwise approach was followed to reach a parsimonious model (model B), in which a main effect analysis was done. Selection of predictors was based on significance shown in an univariate analysis; following variables were tested: age, sex, CCI total score, CCI components, renal function (according to CG and to CKD-EPI), surgical vs internal medicine ward, number of preadmission drugs and the number of preadmission RASP PIMs. Additionally, a low Akaike information criterion (AIC) value was preferred.
A total sample size of 44 patients (i.e. 2 × 22) was estimated to detect a median difference of two recommendations with the Mann Whitney U test, with alpha and beta defined as 0.05 and 0.20 respectively. Due to the practical and explorative nature of the study, we aimed to enrol 30 patients per cohort (usual care and intervention).
All statistical tests were two-tailed and statistical significance was set at p < 0.05. Statistical analysis was performed using SPSS (Statistical Package for Social Sciences; IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp).
Results
Both investigators (JH and LD) showed a favourable reproducible approach in the training set of patient cases, respectively scoring a Cohen’s kappa of 0.72 and 0.73.
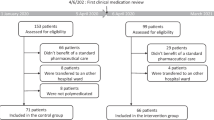
In total, 60 patients were enrolled consecutively of whom 59 were included in the analysis of the primary outcome measure. One patient was excluded from analysis due to the institution of end-of-life care during hospital stay which precluded any IGCT counselling. Detailed information on patient flow through the study is depicted in Fig. 1.
Patient characteristics did not differ significantly between both cohorts. Average age-adjusted CCI was 7.12 (95% confidence interval (CI): 6.39, 7.84). Patients were drawn from 20 nursing wards, covering a total of 14 medical and surgical disciplines. More information has been provided in Table 1. Most common disciplines were the trauma ward (19/59) and cardiology units (15/59). In total, 24 and 35 patients were admitted to a surgical or medical unit, respectively. Twenty-three patients (23/59) were admitted to the hospital due to a fall as the main reason or as one of multiple reasons for admission. There was no difference seen in the number or type of RASP PIMs at baseline in the usual care vs. intervention patients, except a higher frequency of potentially inadequate inhalation in COPD in the intervention cohort (usual care vs. intervention: 0/29 vs 6/30, p = 0.024). Other prevalent pre-admission RASP PIMs have been summarized in Table 2.
In total, 254 drug-related recommendations were provided by the IGCT, of which 94.1% occurred in the intervention cohort. Due to the RASP-based intervention, the number of drug-related recommendations increased from a median of 0 (IQR: 0–1) to 8 (IQR: 6.75–10) per patient (p < 0.001).
The median number of accepted recommendations differed significantly in favor of the intervention cohort group (usual care vs. intervention: 0.0 (0.0–0.5) vs. 3.0 (0.0–5.3), p < 0.001). The median acceptance rate did not differ between both cohorts (usual care vs. intervention: 45% vs 50%, p = 0.140).
At least one IGCT recommendation was adopted into the discharge letter in more patients in the intervention (24/29) than in the usual care cohort (4/29) (p < 0.001). In the intervention cohort, 47.2% (IQR: 33.2–50.0%) of the drug-related recommendations was based on the RASP list, which was significantly more than in usual care patients (0.0%, IQR: 0.0–0.0%) (p < 0.001). Additionally, other drug-related recommendations, that were not based on RASP list, were observed more frequently in intervention patients (median (IQR): usual care vs. intervention: 0.0 (0.0–0.0) vs. 3.0 (2.0–5.0), P < 0.001).
In the intervention cohort, patients were discharged with fewer drugs compared to admission than in the usual care cohort (proportion of drugs on admission, relative to discharge (%): usual care vs. intervention: 108.5%, IQR: 100.0–135.8% vs. 92%, IQR: 80.5–103.5%; p = 0.002). More RASP PIMs were discontinued in intervention patients during hospital stay, i.e. a mean difference was observed of 1.49 RASP PIMs (95% CI: 0.70, 2.23; p < 0.001). Patients in the intervention cohort received furthermore fewer RASP PIMs at discharge (usual care vs. intervention: 2.50, IQR: 2.0–3.8 vs. 1.0, IQR: 0.0–3.0; p = 0.008).
None of the individual RASP PIMs had been significantly discontinued more frequently in the intervention than in the usual care cohort. Trends towards higher discontinuation rates in favour of the intervention were observed for the following RASP items: prolonged use of benzodiazepines, zolpidem, zopiclone or zaleplon; prolonged use of antidepressants; use of inhalation corticosteroids in the treatment of COPD GOLD I-II; duplicate therapy (e.g. two different beta blockers). The content of the non-RASP based recommendations was very diverse (i.e. low prevalence per type of recommendation); they could largely be reduced to clinically relevant medication discrepancies and drug therapies lacking or with unclear indications. More information on drug use has been provided in Table 3.
The Poisson regression analysis identified two significant determinants for the dependent outcome measure of number of IGCT provided drug-related recommendations: application of the systematic medication review in the intervention cohort coincided with an incidence rate ratio (IRR) of 14.1 (95% CI: 8.3, 23.8, p < 0.001) and the number of preadmission drugs with an IRR of 1.06 (95% CI: 1.03, 1.09). No other significant determinants were observed. Model A is the unadjusted model with intervention vs. usual care as the sole determinant. Model B is the adjusted model which shows a small effect of the number of preadmission drugs. Both models have been depicted in Table 4.
Discussion
A before-after study was undertaken in a large teaching hospital to increase the number of drug-related recommendations provided by the IGCT. Enrolled patients were older adults who had been admitted to other than acute geriatric wards and in whom a IGCT consultation had been requested. The intervention on top of usual care provided by the IGCT was able to significantly increase the number of drug-related recommendations. In this study, we found a very low number of drug-related recommendations in the before cohort (usual care). This might largely be explained by nurse IGCT members having been the first and predominant contact with the patients. These nurses were not yet accustomed with performing medication reconciliation and review.
The following secondary outcome measures should be considered exploratory. The criteria of the RASP list accounted for 47.2% (IQR: 33.3–55.0%) of the provided recommendations in the intervention cohort, which is in accordance with our previous experiences with the RASP list [2]. A median of 45% of the recommendations was accepted by the ward-based physicians in the intervention cohort, with a significant uptake of the IGCT recommendations into the final discharge letter. The latter was unexpected, given that drug-related recommendations were added to the patient’s file without further oral communication. Ward-based physicians were however found ready to apply the recommendations, which suggests promising results on the applicability of such interventions. We hypothesize that a different approach, with active publicity within the hospital for this type of additional IGCT service, would probably increase the downstream uptake of the drug-related recommendations.
Our results are in line with those found in a previous investigation, in which the intervention had also been based on the use of the RASP list [2]. Both this and the previous study were performed in a geriatric inpatient population and investigated comparable interventions. Several differences should be noted however. First, patients were enrolled from acute geriatric care wards as opposed to non-geriatric care wards. As a consequence, usual care consisted of geriatricians in the previous study, who were already trained in improving drug therapies. Second, patients were on average 2 years older and suffered from worse renal function. Third, the medication review service at the time was done solely by hospital pharmacists and pharmaceutical services were furthermore provided during the complete hospital stay, i.e. from admission to discharge. Nonetheless, both investigations comprised comparable comorbid patients who took nearly identical numbers of preadmission drugs. More importantly, the interventions in both studies led to similar reductions of RASP PIMs at discharge, which further corroborates the applicability of a RASP-based intervention in older inpatients.
Providing a structured medication review, such as the one investigated in this study, can improve the quality of prescribing and might also impact several clinical outcome measures, such as drug-related readmissions [2, 8, 26, 27]. The evidence remains limited however to a small number of positive studies, many of which involved ward-based pharmacists, who were frequently incorporated into multidisciplinary teams [4, 5, 28]. Pharmacist interventions frequently entail providing a medication reconciliation, a medication review, patient education and follow-up after discharge [28]. As team members, pharmacists offer an additional perspective on how to improve drug therapies in older patients and have been identified as suitable candidates to apply medication reviews [5, 28]. A structured medication review, whether or not involving pharmacists, has however not been rigorously been investigated within the context of a CGA [5]. This specific context might offer distinct advantages as opposed to providing an ad hoc medication review. Not only the medical and social, but also functional capabilities are ascertained within the CGA context, supporting individualized drug-related recommendations; frailty, life expectancy, cognitive reserve among others all play a significant role in determining whether certain drug therapies should be initiated, changed or discontinued [29].
To the best of our knowledge, only Dalleur et al. have investigated a comparable IGCT based approach, in which a systematic medication review was also provided as part of the CGA [20]. Enrolled participants had similar profiles, having a comparable age and overall drug use. Their intervention was performed however by geriatricians of the IGCT, who applied a medication review that was based on the STOPP criteria. Hence, both study designs differ regarding the specific screening tool and involvement of the specific health-care professional (trained geriatricians versus clinical pharmacy/medicine students). They concluded that using an explicit screening tool was operational in different settings, including the decentral IGCT setting, and that the intervention resulted in a doubling of PIM reduction, which is comparable to our results.
Following strengths of our study can be noted. Due to the design no teaching contamination could have occurred. With an additional effort, in this case performed by two healthcare professionals-in-training having been provided with limited training themselves, the IGCT care delivery model was able to significantly impact drug prescribing for older inpatients. Time investment of the investigators was limited as well: medication reconciliation and review if applied systematically was not considered to be overly time consuming (i.e. less than 30 min per patient). The external validity is furthermore reassuring as the main intervention was carried by two junior investigators under the supervision of a senior investigator. Also, our trial results are in line with results gathered in previous investigations [2, 20].
Several limitations should however be taken into account. This was an exploratory before-after study (n = 59), which means that our results might have been overestimated or be explained by unmeasured confounders. Hence, results should be interpreted with caution. Yet, temporal trends would likely be of no influence, as the study was completed in a two-month period within one hospital. Also, other determinants for the primary outcome measure were found to be of no or at best minimal impact in a predefined regression analysis. Lastly, no heterogeneity was observed across multiple outcome measures (e.g. more recommendations, less drugs, less RASP PIMs). Another major limitation is that no clinically relevant patient outcomes were evaluated. A larger and in particular controlled study with less stringent enrolment criteria should hence be undertaken to confirm our findings in a broader inpatient sample and to investigate the impact of the intervention on clinical outcome measures such as adverse drug reactions or unplanned drug-related hospital readmissions. We also did not follow-up patients after discharge, which limits our conclusions regarding the long-term impact of our intervention. Furthermore, not every hospital has access to trained pharmacists or geriatricians to oversee the training of involved health-care professionals, which might limit the external validity of our findings. The latter could however be mitigated by providing education through e-learning [30, 31].
Finally, the following issues should be targeted in future investigations. First, trained pharmacists can play an important role in an IGCT approach and have only been involved infrequently in this specific setting as can be inferred from the scarcity in the literature. Second, further studies should investigate whether ward-based nurses could undertake a relevant role in the medication review process by screening for potentially inappropriate therapies or aiding and implementing therapy changes for potentially inappropriate therapies or aiding in implementing therapy changes [32]. Previous investigations in different settings have already shown that trained nurses can have a beneficial impact on drug use, e.g. in the outpatient follow-up of heart failure patients or the management of atrial fibrillation patients, both settings with a high degree of polypharmacy [33, 34]. Third, CDSS might play an important role in expanding medication review services in the hospital setting and decreasing therapeutic inertia (e.g. alerting to the inappropriate use of sotalol in heart failure patients, or the use of flecainide in patients with previous coronary ischemia). We eagerly await the results of two ongoing European trials, OPERAM and SENATOR, both incorporating software packages to manage inappropriate drug use in older hospitalized adults [35]. Fourth, more studies should be performed to further confirm the benefits of complementing CGA with structured medication reviews.
Conclusions
Adding a systematic medication review to a CGA performed by the IGCT may have benefits. A large association was seen in this before-after study between providing a systematic medication review and an increase of drug-related recommendations provided by the IGCT. Regression analysis identified the number of preadmission drugs to be another potential determinant for the number of drug-related recommendations. More RASP PIMs were discontinued during hospital stay in the intervention cohort, which coincided with a relative decrease in the number of drugs. Due to the quasi-experimental design, results should be interpreted with caution.
Abbreviations
- AIC:
-
Akaike information criterion
- CGA:
-
Comprehensive geriatric assessment
- CI:
-
Confidence interval
- CrCL CG:
-
Estimated creatinine clearance by the Cockcroft-Gault equation
- eGFR CKD-EPI:
-
Estimated Glomerular Filtration Rate by the Chronic Kidney Disease Epidemiology (Collaboration) equation
- IGCT:
-
Interdisciplinary geriatric consultation team
- IQR:
-
Interquartile range
- IRR:
-
Incidence rate ratio
- PIM:
-
Potentially inappropriate medication
- RASP:
-
Rationalization of Home Medication by an Adjusted STOPP in Older Patients
- SD:
-
Standard deviation
References
Renaudin P, Boyer L, Esteve MA, Bertault-Peres P, Auquier P, Honore S. Do pharmacist-led medication reviews in hospitals help reduce hospital readmissions? A systematic review and meta-analysis. Br J Clin Pharmacol. 2016;82(6):1660–73.
Van der Linden L, Decoutere L, Walgraeve K, Milisen K, Flamaing J, Spriet I, Tournoy J. Combined use of the rationalization of home medication by an adjusted STOPP in older patients (RASP) list and a pharmacist-led medication review in very old inpatients: impact on quality of prescribing and clinical outcome. Drugs Aging. 2017;34(2):123–33.
O'Sullivan D, O'Mahony D, O'Connor MN, Gallagher P, Gallagher J, Cullinan S, O'Sullivan R, Eustace J, Byrne S. Prevention of adverse drug reactions in hospitalised older patients using a software-supported structured pharmacist intervention: a cluster randomised controlled trial. Drugs Aging. 2016;33(1):63–73.
Christensen M, Lundh A. Medication review in hospitalised patients to reduce morbidity and mortality. Cochrane Database Syst Rev. 2016;2:Cd008986.
Petrovic M, Somers A, Onder G. Optimization of geriatric pharmacotherapy: role of multifaceted cooperation in the hospital setting. Drugs Aging. 2016;33(3):179–88.
Hohl CM, Wickham ME, Sobolev B, Perry JJ, Sivilotti ML, Garrison S, et al. The effect of early in-hospital medication review on health outcomes: a systematic review. Br J Clin Pharmacol. 2015;80(1):51–61.
Gillespie U, Alassaad A, Hammarlund-Udenaes M, Morlin C, Henrohn D, Bertilsson M, Melhus H. Effects of pharmacists’ interventions on appropriateness of prescribing and evaluation of the instruments' (MAI, STOPP and STARTs') ability to predict hospitalization—analyses from a randomized controlled trial. PLoS One. 2013;8:e62401.
Spinewine A, Swine C, Dhillon S, Lambert P, Nachega JB, Wilmotte L, Tulkens PM. Effect of a collaborative approach on the quality of prescribing for geriatric inpatients: a randomized, controlled trial. J Am Geriatr Soc. 2007;55(5):658–65.
Borne R, Cumbler E, Glasheen JJ. Reducing polypharmacy: is hospitalization the right time? Arch Intern Med. 2011;171:869. author reply 870
Linnebur SA, O'Connell MB, Wessell AM, McCord AD, Kennedy DH, DeMaagd G, Dent LA, Splinter MY, Biery JC Jr, Chang F, et al. Pharmacy practice, research, education, and advocacy for older adults. Pharmacotherapy. 2005;25(10):1396–430.
Renaudin P, Baumstarck K, Daumas A, Esteve MA, Gayet S, Auquier P, Tsimaratos M, Villani P, Honore S. Impact of a pharmacist-led medication review on hospital readmission in a pediatric and elderly population: study protocol for a randomized open-label controlled trial. Trials. 2017;18(1):65.
Pellegrin KL, Krenk L, Oakes SJ, Ciarleglio A, Lynn J, McInnis T, Bairos AW, Gomez L, McCrary MB, Hanlon AL, et al. Reductions in medication-related hospitalizations in older adults with medication management by hospital and community pharmacists: a quasi-experimental study. J Am Geriatr Soc. 2017;65(1):212–9.
Van Der Linden L, Decoutere L, Flamaing J, Spriet I, Willems L, Milisen K, Boonen S, Tournoy J. Development and validation of the RASP list (rationalization of home medication by an adjusted STOPP list in older patients): a novel tool in the management of geriatric polypharmacy. Eur Geriatr Med. 2014;5(3):175–80.
Deschodt M, Braes T, Broos P, Sermon A, Boonen S, Flamaing J, Milisen K. Effect of an inpatient geriatric consultation team on functional outcome, mortality, institutionalization, and readmission rate in older adults with hip fracture: a controlled trial. J Am Geriatr Soc. 2011;59(7):1299–308.
Ellis G, Gardner M, Tsiachristas A, Langhorne P, Burke O, Harwood RH, Conroy SP, Kircher T, Somme D, Saltvedt I, et al. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev. 2017;9:Cd006211.
Pilotto A, Cella A, Daragjati J, Veronese N, Musacchio C, Mello AM, Logroscino G, Padovani A, Prete C, Panza F. Three decades of comprehensive geriatric assessment: evidence coming from different healthcare settings and specific clinical conditions. J Am Med Dir Assoc. 2017;18(2):192. e191–192.e111
Van Grootven B, Flamaing J, Dierckx de Casterle B, Dubois C, Fagard K, Herregods MC, Hornikx M, Laenen A, Meuris B, Rex S, et al. Effectiveness of in-hospital geriatric co-management: a systematic review and meta-analysis. Age Ageing. 2017;46(6):903–10.
Cameron ID, Kurrle S. Geriatric consultation services-are wards more effective than teams? BMC Med. 2013;11:49.
Deschodt M, Flamaing J, Haentjens P, Boonen S, Milisen K. Impact of geriatric consultation teams on clinical outcome in acute hospitals: a systematic review and meta-analysis. BMC Med. 2013;11:48.
Dalleur O, Boland B, Losseau C, Henrard S, Wouters D, Speybroeck N, Degryse JM, Spinewine A. Reduction of potentially inappropriate medications using the STOPP criteria in frail older inpatients: a randomised controlled study. Drugs Aging. 2014;31(4):291–8.
Deschodt M, Claes V, Van Grootven B, Van den Heede K, Flamaing J, Boland B, Milisen K. Structure and processes of interdisciplinary geriatric consultation teams in acute care hospitals: a scoping review. Int J Nurs Stud. 2016;55:98–114.
Braes T, Moons P, Lipkens P, Sterckx W, Sabbe M, Flamaing J, Boonen S, Milisen K. Screening for risk of unplanned readmission in older patients admitted to hospital: predictive accuracy of three instruments. Aging Clin Exp Res. 2010;22(4):345–51.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83.
De Winter S, Vanbrabant P, Spriet I, Desruelles D, Indevuyst C, Knockaert D, Gillet JB, Willems L. A simple tool to improve medication reconciliation at the emergency department. Eur J Intern Med. 2011;22(4):382–5.
Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–74.
Gillespie U, Alassaad A, Henrohn D, Garmo H, Hammarlund-Udenaes M, Toss H, Kettis-Lindblad A, Melhus H, Morlin C. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older: a randomized controlled trial. Arch Intern Med. 2009;169:894–900.
Gallagher PF, O'Connor MN, O'Mahony D. Prevention of potentially inappropriate prescribing for elderly patients: a randomized controlled trial using STOPP/START criteria. Clin Pharmacol Ther. 2011;89:845–54.
Spinewine A, Fialova D, Byrne S. The role of the pharmacist in optimizing pharmacotherapy in older people. Drugs Aging. 2012;29(6):495–510.
Benetos A, Rossignol P, Cherubini A, Joly L, Grodzicki T, Rajkumar C, Strandberg TE, Petrovic M. Polypharmacy in the aging patient: Management of Hypertension in octogenarians. JAMA. 2015;314(2):170–80.
Franchi C, Tettamanti M, Djade CD, Pasina L, Mannucci PM, Onder G, Gussoni G, Manfellotto D, Bonassi S, Salerno F, et al. E-learning in order to improve drug prescription for hospitalized older patients: a cluster-randomized controlled study. Br J Clin Pharmacol. 2016;82(1):53–63.
Keijsers CJ, van Doorn AB, van Kalles A, de Wildt DJ, Brouwers JR, van de Kamp HJ, Jansen PA. Structured pharmaceutical analysis of the systematic tool to reduce inappropriate prescribing is an effective method for final-year medical students to improve polypharmacy skills: a randomized controlled trial. J Am Geriatr Soc. 2014;62(7):1353–9.
Dilles T, Vander Stichele RH, Van Bortel LM, Elseviers MM. The development and test of an intervention to improve ADR screening in nursing homes. J Am Med Dir Assoc. 2013;14(5):379. e371–376
Hendriks JM, de Wit R, Crijns HJ, Vrijhoef HJ, Prins MH, Pisters R, Pison LA, Blaauw Y, Tieleman RG. Nurse-led care vs. usual care for patients with atrial fibrillation: results of a randomized trial of integrated chronic care vs. routine clinical care in ambulatory patients with atrial fibrillation. Eur Heart J. 2012;33(21):2692–9.
Bowyer JL, Tully PJ, Ganesan AN, Chahadi FK, Singleton CB, McGavigan AD. A randomised controlled trial on the effect of nurse-led educational intervention at the time of catheter ablation for atrial fibrillation on quality of life, symptom severity and rehospitalisation. Heart Lung Circ. 2017;26(1):73–81.
Lavan AH, Gallagher PF, O'Mahony D. Methods to reduce prescribing errors in elderly patients with multimorbidity. Clin Interv Aging. 2016;11:857–66.
Acknowledgements
The authors would like to thank all IGCT members, as they were all indirectly involved in performing this study. In particular, we wish to acknowledge the assistance of Katleen Fagard of the Department of Geriatric Medicine, University Hospitals Leuven, Louvain, Belgium.
Funding
This investigation (design and execution) was not funded. LVDL received a partial research scholarship of the University Hospitals Leuven.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to the protection of the enrolled participants’ privacy, but are available (in anonymized manner) from the corresponding author upon reasonable request.
Author information
Authors and Affiliations
Contributions
LVDL, JH and LD participated in the study concept and design, acquisition of subjects and data, analysis and interpretation of data, and preparation of manuscript. JT, IS and JF participated in the study concept and design, analysis of data and preparation of the manuscript. KM participated in the interpretation of data and preparation of the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The trial design was approved the Ethics Committee of the University Hospitals Leuven, Belgium (reference number: ML9908). Patients were considered for inclusion if written informed consent was provided by the patients, or by their relatives in case of them being unable to provide consent.
Consent for publication
Not applicable.
Competing interests
Two authors (KM and JF) are members of the editorial board of BMC Geriatrics. The authors declare that they have no further competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Van der Linden, L., Hias, J., Dreessen, L. et al. Medication review versus usual care to improve drug therapies in older inpatients not admitted to geriatric wards: a quasi-experimental study (RASP-IGCT). BMC Geriatr 18, 155 (2018). https://doi.org/10.1186/s12877-018-0843-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12877-018-0843-y