Abstract
Background
Indigo naturalis is a Chinese herbal medicine that has currently been used to treat various inflammatory diseases, including ulcerative colitis. Recently, there are several reports concerning severe adverse events associated with indigo naturalis.
Case presentation
We described a case of a 44-year-old female with ulcerative colitis who presented with lower abdominal pain and hematochezia. She stopped taking her medicine for ulcerative colitis and started oral indigo naturalis 3 months before admission. Computed tomography showed segmental edematous wall thickening of the descending and sigmoid colon. Colonoscopy findings revealed erythema, edema, and submucosal hemorrhage, the surface of which presented a dark blue pigmentation. The histologic finding was consistent with ischemic colitis. We therefore considered an ischemic colitis induced by indigo naturalis, and the patient improved after supportive care and withdrawal of indigo naturalis.
Conclusion
Indigo naturalis has currently been used in the patients with ulcerative colitis as an alternative therapy. However, physicians should be aware of possible severe adverse events such as ischemic colitis.
Similar content being viewed by others
Background
Indigo naturalis (also known as Qing-dai) is a Chinese herbal medicine with a dark blue color, which is extracted from the leaves and stems of plants such as Indigofera tinctoria, Strobilanthes cusia O Kuntze, and Polygonum tinctorium Lour. Indigo naturalis has been used in China for centuries to treat various inflammatory diseases [1, 2].
There have recently been several reports suggesting that indigo naturalis is effective in patients with active ulcerative colitis, although the evidence regarding efficacy and safety is still limited [3,4,5,6]. Common adverse effects of indigo naturalis are liver dysfunction, headache, abdominal pain, and nausea [3,4,5,6]. Recently, there have been concerns about severe adverse events like pulmonary arterial hypertension and acute colitis in patients taking indigo naturalis [7,8,9,10,11,12].
We herein report a patient with ulcerative colitis who developed ischemic colitis during oral administration of indigo naturalis.
Case presentation
A 44-year-old woman with a two-year history of ulcerative colitis was admitted to our hospital with complaints of lower abdominal pain and hematochezia. The patient had previously been treated with oral mesalazine and intermittent corticosteroids at another hospital, and the disease extent was the extensive colitis involved to the transverse colon. The patient had no other medical history, including hypertension, diabetes, cardiovascular disease, chronic kidney disease or abdominal surgery.
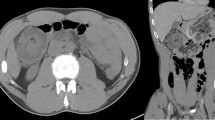
Three months prior to admission, she independently stopped taking her medication for ulcerative colitis and started taking self-purchased oral indigo naturalis twice a day (daily dose, 2 g). She had no history of taking other medication. After 2 months of taking the oral indigo naturalis, she developed intermittent hematochezia. Two weeks later, she exhibited lower abdominal pain. The patient’s vital signs were stable on admission. Her physical examination revealed tenderness on lower abdomen, but not rebound tenderness. Laboratory examination on admission showed a white blood cell count of 9.4 × 103/μL (reference, 4000-10,000/μL), hemoglobin level of 13.2 g/dL (reference, 12–16 g/dL for female), and C-reactive protein concentration of 0.45 mg/dL (reference, < 0.3 mg/dL). A stool culture and Clostridium difficile toxin study were both negative. Computed tomography of abdomen and pelvis showed segmental edematous wall thickening of the descending and sigmoid colon, and no abnormal finding of mesenteric vessels (Fig. 1). Colonoscopy findings revealed erythema, edema, and submucosal hemorrhage, the surface of which presented a dark blue pigmentation with the same color as the original indigo naturalis in the descending and sigmoid colon, but a relatively non-inflamed finding in the rectum (Fig. 2a, b). Biopsy specimens from the descending and sigmoid colon showed erosion and loss of superficial epithelium with few inflammatory infiltrations, which is histologically consistent with ischemic colitis rather than a flare-up of ulcerative colitis or other colitis such as infectious colitis (Fig. 3a, b). Based on these findings, we considered the patient to have ischemic colitis induced by indigo naturalis. Supportive care with bowel rest, intravenous hydration, empirical antibiotics, and discontinuation of indigo naturalis resulted in improvement within a week. She then agreed to be treated with prednisolone and mesalazine for ulcerative colitis, and a follow-up colonoscopy 2 months later showed an improved state with some scar changes and an indigo blue pigmentation still remaining on the colon (Fig. 4a, b).
Discussion and conclusion
Indigo naturalis has been considered mainly as an anti-fever and anti-inflammatory agent in Chinese textbooks since the tenth century, and has therefore been used to treat various inflammatory diseases for centuries [1, 2]. There have recently been studies suggesting the efficacy of indigo naturalis in patients with active ulcerative colitis [3,4,5,6]. The actual mechanisms of action of indigo naturalis still remain unknown, but one possible mechanism is that the active components of indigo naturalis can activate the aryl hydrocarbon receptor (AhR) by acting as an AhR ligand, and promote mucosal healing by the upregulation of interleukin-22 from innate lymphocytes cells that express AhR [2, 13].
The first study to evaluate the therapeutic efficacy of oral indigo naturalis in patients with intractable ulcerative colitis showed that clinical activity index scores and endoscopic grades decreased in the patients taking 2 g of indigo naturalis daily for 4 months [3]. In 2018, Naganuma et al. conducted a randomized controlled trial in Japan to investigate the clinical efficacy and safety of indigo naturalis in patients with moderate to severe ulcerative colitis [5]. Total of 86 patients were enrolled and randomized for each group, the results indicated that 8 weeks of oral indigo naturalis produced a significant clinical response in the indigo naturalis groups. The response rates at week 8 were 69.6% (16/23, 0.5 g/day group), 75.0% (15/20, 1.0 g/day group), 81.0% (17/21, 2.0 g/day group), and 13.6% (3/22, placebo group), respectively. However, this trial was terminated early because a case of pulmonary arterial hypertension was reported in a patient with ulcerative colitis who had used self-purchased indigo naturalis outside of this study [5, 7].
Some patients have recently started using indigo naturalis obtained over the Internet, but these patients do not always receive sufficient information regarding the adverse effects caused by indigo naturalis. Common mild adverse events of indigo naturalis include liver dysfunction, headache, epigastric or abdominal pain, and nausea [3,4,5,6]. Rare severe adverse events associated with indigo naturalis have included indigo naturalis-induced pulmonary arterial hypertension and acute colitis [7,8,9,10,11,12]. Nishio et al. reported the development of pulmonary arterial hypertension in a patient with ulcerative colitis who was taking indigo naturalis [7]. An experimental study suggested that a possible mechanism for triggering indigo naturalis-induced pulmonary arterial hypertension involves nitric oxide synthase inhibition and endothelial dysfunction in the pulmonary artery [8]. Recently, indigo naturalis-induced colitis has also been reported. A prospective study about clinical efficacy and safety of indigo naturalis in patients with ulcerative colitis described right-side colitis of obscure origin [4]. Kondo et al. also reported two patients with ulcerative colitis who developed colitis with wall thickening and edematous changes during oral administration of indigo naturalis [10].. In addition, Matsuno et al. reported two patients who developed phlebitis-induced colitis caused by indigo naturalis. They suggested that indigo naturalis might affect the venous system and cause adverse events such as phlebitis-induced colitis or pulmonary artery hypertension [12].
We herein described a case of ischemic colitis in a patient with ulcerative colitis after taking indigo naturalis. About 30 to 50% of patients with inflammatory bowel disease are known to use complementary and alternative medicines [14]. Thus, detailed medical history is needed when treating patients with inflammatory bowel disease. Indigo naturalis seems to be another therapeutic candidate for managing ulcerative colitis, but more research is needed to determine the optimal dose, standardized drug development, and indications for treatment. In addition, physicians should be aware of the possible risks of severe adverse events. Further basic studies and clinical investigation are needed to clarify the mechanisms of these possible adverse events of indigo naturalis.
Availability of data and materials
This case report contains clinical data from the electronic medical record in the Chungbuk National University Hospital. Additional information is available from the corresponding author on reasonable request from the editor.
Abbreviations
- AhR:
-
Aryl hydrocarbon receptor
References
Sugimoto S, Naganuma M, Kanai T. Indole compounds may be promising medicines for ulcerative colitis. J Gastroenterol. 2016;51(9):853–61.
Naganuma M. Treatment with indigo naturalis for inflammatory bowel disease and other immune diseases. Immunol Med. 2019;42(1):16–21.
Suzuki H, Kaneko T, Mizokami Y, Narasaka T, Endo S, Matsui H, et al. Therapeutic efficacy of the Qing Dai in patients with intractable ulcerative colitis. World J Gastroenterol. 2013;19(17):2718–22.
Sugimoto S, Naganuma M, Kiyohara H, Arai M, Ono K, Mori K, et al. Clinical efficacy and safety of oral Qing-Dai in patients with ulcerative colitis: a single-center open-label prospective study. Digestion. 2016;93(3):193–201.
Naganuma M, Sugimoto S, Mitsuyama K, Kobayashi T, Yoshimura N, Ohi H, et al. Efficacy of indigo naturalis in a multicenter randomized controlled trial of patients with ulcerative colitis. Gastroenterology. 2018;154(4):935–47.
Urushikubo J, Yanai S, Nakamura S, Kawasaki K, Akasaka R, Sato K, et al. Efficacy of indigo naturalis therapy for ulcerative colitis: a case series. Intern Med. 2019;58(16):2299–304.
Nishio M, Hirooka K, Doi Y. Chinese herbal drug natural indigo may cause pulmonary artery hypertension. Eur Heart J. 2016;37(25):1992.
Sato K, Ohira H, Horinouchi T, Nakaya T, Mazaki Y, Sugimoto A, et al. Chinese herbal medicine Qing-Dai-induced pulmonary arterial hypertension in a patient with ulcerative colitis: a case report and experimental investigation. Respir Med Case Rep. 2019;26:265–9.
Misumi K, Ogo T, Ueda J, Tsuji A, Fukui S, Konagai N, et al. Development of pulmonary arterial hypertension in a patient treated with qing-dai (Chinese herbal medicine). Intern Med. 2019;58(3):395–9.
Kondo S, Araki T, Okita Y, Yamamoto A, Hamada Y, Katsurahara M, et al. Colitis with wall thickening and edematous changes during oral administration of the powdered form of Qing-dai in patients with ulcerative colitis: a report of two cases. Clin J Gastroenterol. 2018;11(4):268–72.
Yanai S, Nakamura S, Matsumoto T. Indigo naturalis-induced colitis. Dig Endosc. 2018;30(6):791.
Matsuno Y, Hirano A, Esaki M. Possible association of phlebitis-induced colitis with indigo naturalis. Gastroenterology. 2018;155(2):576–7.
Kawai S, Iijima H, Shinzaki S, Hiyama S, Yamaguchi T, Araki M, et al. Indigo naturalis ameliorates murine dextran sodium sulfate-induced colitis via aryl hydrocarbon receptor activation. J Gastroenterol. 2017;52(8):904–19.
Cheifetz AS, Gianotti R, Luber R, Gibson PR. Complementary and alternative medicines used by patients with inflammatory bowel diseases. Gastroenterology. 2017;152(2):415–29.
Acknowledgements
We would like to acknowledge our patient for her support.
Funding
There are no funding sources for this case report.
Author information
Authors and Affiliations
Contributions
BC and SMY performed the literature review and collected the clinical data. BC prepared the first manuscript. All authors (BC, SMY, SMS, HWK, KBK and SJY) participated in further drafting and revision of the manuscript. Finally, all authors (BC, SMY, SMS, HWK, KBK and SJY) have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Authors’ institution does not require ethical approval for publication of a single case report. Written informed consent was obtained from the patient.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and the accompanying images. A copy of the written consent is available upon request.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Cho, B., Yoon, S.M., Son, SM. et al. Ischemic colitis induced by indigo naturalis in a patient with ulcerative colitis: a case report. BMC Gastroenterol 20, 154 (2020). https://doi.org/10.1186/s12876-020-01301-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-020-01301-3