Abstract
Background
N-terminal pro-B-type natriuretic peptide (NT-proBNP) is a potential biomarker for monitoring the status of heart failure. However, the optimal monitoring interval of NT-proBNP is unknown. This study sought to investigate the minimal informative monitoring interval of NT-proBNP in patients with stable chronic heart failure.
Methods
This retrospective cohort study included patients who were admitted due to heart failure and subsequently followed with serial NT-proBNP measurements in a tertiary hospital. We analyzed NT-proBNP measured between six months after discharge and the earliest timepoint of: an alteration of medication regimen, readmission due to worsening of heart failure, or all-cause death. To distinguish progression of the disease from biological variability and measurement error, the signal-to-noise ratio method was applied with a random-effects model.
Results
In the 368 patients included, NT-proBNP was measured for a median 6 times. In the random-effects model, signal (progression of disease) exceeded noise (biological variability and measurement error) at 7.9 months (95% confidence interval [CI]: 5.1–9.6), while noise corresponded to a 61% increase from baseline. In stratified analysis using the AHEAD risk score, the minimal informative monitoring interval shortened as the risk score increased (0–1 point: 12.2 months [95%CI: 10.3–14.4]; 2–3 points: 8.0 months [95%CI: 6.8–9.7]; 4–5 points: 3.3 months [95%CI: 3.0–3.8]).
Conclusions
In patients with stable chronic heart failure, the minimal informative monitoring interval of NT-proBNP measurement was 7.9 months in the current population, which varied with underlying risks. The optimal monitoring interval could be lengthened for patients at lower risks.
Similar content being viewed by others

Background
Biochemical markers, including natriuretic peptides such as N-terminal pro-B-type natriuretic peptide (NT-proBNP), are widely used in the clinical management of heart failure. The serum level of NT-proBNP can reliably be used as a diagnostic tool and a prognostic predictor throughout the wide spectrum of acute and chronic heart failure [1,2,3,4,5]. Moreover, recent studies reported potential utility of NT-proBNP for monitoring chronic heart failure and titrating medical therapy [6,7,8]. However, this still remains controversial [9,10,11,12], and no established consensus has been reached regarding the monitoring interval of NT-proBNP in patients with chronic heart failure.
Several studies have reported that serum NT-proBNP was measured routinely every 3 months upon follow-up visits in clinical management of chronic heart failure [9, 12]. Nevertheless, NT-proBNP measurement is susceptible to up to 40% of intra-individual variability without disease progression, which hinders the detection of a true change in the disease status [13,14,15,16]. Frequent measurements, therefore, may lead to therapeutic confusion as well as unnecessary costs. Thus, it is crucial to clarify the minimal informative interval for monitoring NT-proBNP in these patients. The purpose of the present study is to investigate the minimal informative interval for testing serum NT-proBNP level in patients with stable heart failure using real-world data, taking the intra-individual variability into account.
Methods
Study design
A single-center retrospective open-cohort study was conducted to identify the true progression and intra-individual variability, as well as to investigate a minimal informative interval of NT-proBNP measurement in patients with stable chronic heart failure. The present study was approved by the institutional research board of St. Luke’s International University (NO. 18-R070). All patients provided informed consent under an opt-out policy.
Study population
Consecutive adult patients (aged 20 or more) who had been hospitalized at St. Luke’s International Hospital (Tokyo, Japan) with a diagnosis of heart failure from January 2003 to December 2017 were assessed for enrollment in the present study. The diagnosis of heart failure was based on Framingham criteria [17]. The following patients were excluded: patients 1) who had readmissions for heart failure within 6 months after the index admission; 2) with less than 3 times of NT-proBNP measurement during the analytic period defined below; 3) without any record of left ventricular ejection fraction assessed on transthoracic echocardiography at the time of index admission; 4) with end-stage renal disease requiring either hemodialysis or peritoneal dialysis; 5) who declined the consent. Those patients who had unplanned visits within 6 months after the index admission but were not readmitted, were not excluded. For patients who had multiple admissions due to heart failure during the study period, the first was registered as the index admission.
Definition of analytic period in the present study
We excluded the NT-proBNP measurements acquired in the first 6 months after the index admission in order to allow a window period for stabilization of both heart failure and NT-proBNP [18]. We defined the first measurement of NT-proBNP after 6 months of the index admission as the baseline (i.e. the start of the analytic period). The endpoint of the analytic period was defined as the earliest timepoint at which either a physician changed the regimen of medication for heart failure, when the patient was readmitted for heart failure, or when the patient died from any cause (Online Supplemental Fig. 1). The medication for heart failure included beta-blockers, angiotensin converting enzyme inhibitors, angiotensin receptor blockers, diuretics (thiazides, loop diuretics, mineralocorticoid receptor antagonists, and vasopressin V2-receptor antagonists), digoxin, and pimobendan. As this was a retrospective study, the schedules of outpatient visits and NT-proBNP measurements were left to each physician’s discretion.
Data collection
Clinical and demographic data were collected from the patients’ electronic medical records. They included demographic data (gender, age, body weight, and height), comorbidities (hypertension, diabetes mellitus, dyslipidemia, ischemic heart disease, valvular disease, and kidney disease), smoking status, laboratory data, echocardiographic data, and prescriptions at each outpatient visit. Analyzed laboratory tests were all measured within the study site, and serum NT-proBNP was measured with an ECLusys NT-proBNP II kit (Roche Diagnostics Japan, Inc., Tokyo, Japan). All data were collected from electronic medical records by 2 healthcare information technicians and an independent physician who were not involved in the treatment and care of any of the participating patients.
Statistical analysis
Continuous variables are presented as the median (interquartile range [IQR]) or the mean (standard deviation [SD]) as appropriate. Categorical variables are presented as number (percentage). Statistical analysis and model fitting were performed and visualized using R 3.5.2 with the R packages of “dplyr”, “lme4”, “performance”, “ggplot2”, and “forestplot” [19].
Random-effects model and signal-to-noise ratio
In order to distinguish true progression of NT-proBNP from intra-individual variability, we applied the signal-to-noise ratio method with a random-effects linear regression model [20,21,22]. The model was previously demonstrated of good performance that was similar with a direct method (without modeling) of estimation, in studies investigating monitoring intervals of lipids and glycated hemoglobin [20,21,22]. Log transformation of NT-proBNP was used for model fitting, since in the study population the distribution of NT-proBNP had significant right skewness (Fig. 1) that was consistent with a previous report [23]. The response of the model (Yit in Table 1) was {log (NT-proBNP) – log (baseline NT-proBNP)}. The model with random slopes and random intercepts assumed that each patient could have a different rate of increase over time. As covariates we included age, gender, year of the index admission, body mass index, smoking status, estimated glomerular filtration rate (eGFR) upon the admission, left ventricular ejection fraction measured on echocardiography during the admission, history of admission due to heart failure before the index admission, and comorbidity of hypertension, diabetes, dyslipidemia, atrial fibrillation, ischemic heart disease, and valvular disease, based on clinical significance [24,25,26].
From the model above, we calculated between-individual variability (defined as signal) caused by different rates of increase as the variance of the random slope over time multiplied by time, and intra-individual variability (defined as noise) as the variance of the random residual. Equations in detail are shown in Table 1. Based on previous reports, the minimal informative interval was defined as the time when signal exceeds noise [20,21,22]. We calculated 95% confidence intervals (CI) for the informative intervals through non-parametric bootstrapping (1000 resampling with replacement, 1000 times) [27].
Stratified analysis according to underlying risks
Stratified analysis was performed according to age, gender, body mass index, left ventricular ejection fraction, renal function, presence of a history of multiple admissions due to heart failure, and the AHEAD risk score (0–1, 2–3, and 4–5). The AHEAD risk score comprises atrial fibrillation, hemoglobin < 13.0 g/dL in male or < 12.0 g/dL in female, elderly (> 70 years), abnormal renal function (creatinine > 1.47 mg/dL), and diabetes mellitus, with one point for each item [28].
Each subgroup was fitted to the model described above in order to calculate the signal, noise, and minimal informative monitoring interval.
Results
Participants and baseline characteristics
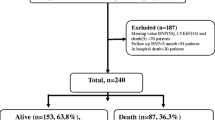
Between January 2003 and December 2017, 1715 adult patients were admitted due to heart failure. Of those patients, 368 patients without end-stage renal disease had 3 or more NT-proBNP measurements during the analytic period (Fig. 2 and Online Supplemental Fig. 1). All the serial NT-proBNP measurements were acquired between July 2009 and December 2017, since NT-proBNP measurement was available from July 2009 at the study site. The median analytic period was 12.0 months (IQR: 6.0–27.0). NT-proBNP was measured for a median 6 times (IQR 4.0–10.0) during the analytic period. The average of the individual mean measurement interval was 2.4 ± 2.4 months.
The median age of included patients was 75.5 years (IQR: 63.0–83.0), of whom 57% were male and 13% had a history of admission due to heart failure before the index admission. The median eGFR was 58.6 mL/min/1.73 m2 (IQR: 45.0–72.9), and the median left ventricular ejection fraction was 43.7% (IQR: 29.0–60.7). The patient baseline characteristics are summarized in Table 2 and the distributions of baseline serum NT-proBNP levels are illustrated in Fig. 1.
Signal, noise, and minimal informative intervals calculated from the random-effects model
The fit of the random-effects model was substantial, with an intraclass correlation coefficient of 0.87 and a conditional r2 of 0.87 [29]. From the estimates of the random-effects model, we obtained the curves of signal and noise of NT-proBNP measurements (Fig. 3 and Online Supplemental Table 1). The minimal informative interval, at which point signal exceeds noise, was 7.9 months (95%CI: 5.1–9.6) in the current population. The standard deviation of εit was 0.48, whose natural exponent was 1.61 (i.e. 161%), corresponding to a 61% increase of NT-proBNP from baseline.
Fig. 4 shows the minimal informative intervals for each stratification by age, gender, body mass index, left ventricular ejection fraction, renal function, history of heart failure admission before the index admission, and AHEAD score. Notably, as the AHEAD risk score increased, the minimal informative interval shortened significantly from 12.2 months (95%CI: 10.3–14.4) in those with scores of 0 or 1, to 3.3 months (95%CI: 3.0–3.8) in those with scores of 4 or 5. The patients with a left ventricular ejection fraction ≥40% had a shorter minimal informative interval than the patients with a left ventricular ejection fraction < 40% (5.2 months [95%CI: 4.5–6.2] vs 12.8 months [95%CI: 7.8–14.5]).
Minimal informative intervals of monitoring NT-proBNP in stable heart failure patients stratified by characteristics and risk. Patients with a preserved ejection fraction (left ventricular ejection fraction [LVEF] ≥ 40%) have a shorter informative interval than those with a reduced ejection fraction (LVEF < 40%). The informative interval decreases markedly as the AHEAD score increases. However, informative intervals do not differ significantly when stratified by age, gender, body mass index, and history of admissions due to heart failure before the index admission. eGFR estimated glomerular filtration rate, HF heart failure, CI confidence interval
Discussion
In the present study analyzing the retrospective data of patients with stable chronic heart failure, a minimal informative interval for monitoring serum NT-proBNP was 7.9 months (95%CI: 5.1–9.6) with a noise corresponding to a 61% change from baseline. More importantly, the minimal informative monitoring interval varied in accordance with different underlying risks. To the best of our knowledge, this is the first attempt to provide an evidence suggesting the informative monitoring interval of NT-proBNP in patients with stable chronic heart failure.
Minimal informative monitoring interval of NT-proBNP taking into account intra-individual variability of NT-proBNP in stable chronic heart failure
NT-proBNP secretion levels are dynamic and depend on the different workloads [13]. Some small physiological stimuli, such as changes in sodium intake or physical exercise before blood test, may substantially influence serum NT-proBNP concentration measured in the outpatient clinic, resulting in biological variability [13]. This, along with measurement error, contributes to large-scale intra-individual variability, suggesting that frequent measurements are more susceptible to noise than to signal [13,14,15,16]. The present study indicates that the intra-individual fluctuation corresponds to a 61% change in the serum NT-proBNP level from baseline. This highlights the limited reliability of a single point measurement of NT-proBNP in the outpatient clinic, emphasizing the importance of other findings derived from symptoms, physical examination, or other tests such as echocardiography. Based on these results, a physician could be recommended in outpatient visits to place NT-proBNP orders after assessing other findings of the patient, and to avoid frequent NT-proBNP tests. While the minimal informative interval of 7.9 months from this study, an optimal monitoring interval of NT-proBNP measurement should be individualized according to the underlying risk profile, and be modified taking the test availability into account. Over-testing might lead to not only therapeutic confusion which might result in over-reaction to noise but also a huge amount of unnecessary costs, especially in healthcare contexts which allow liberal reimbursement for testing. Further studies investigating the financial impact of the minimal informative monitoring interval of NT-proBNP are warranted.
Monitoring interval of NT-proBNP in NT-proBNP-guided therapy
Based on the results of the present study, increasing the length of the monitoring interval of NT-proBNP should be considered in heart failure patients with a stable status without changes in medication. However, in the midst of titration, the optimal monitoring interval of NT-proBNP is more debatable, especially in the case of NT-proBNP-guided therapy. NT-proBNP used as guidance might need to be monitored with a shorter interval due to a likely larger signal-to-noise ratio caused by intensification of treatment. The results of the present study could not be directly applied in such situation. In the Trial of Intensified vs Standard Medical Therapy in Elderly Patients with Congestive Heart Failure (TIME-CHF), Can Pro-brain-natriuretic Peptide Guided Therapy of Chronic Heart Failure Improve Heart Failure Morbidity and Mortality (PRIMA), and Guiding Evidence-based Therapy Using Biomarker-intensified Treatment in Heart Failure (GUIDE-IT) studies that failed to demonstrate prognostic superiority of NT-proBNP-guided therapy over conventional strategies in heart failure patients, patients were followed up every 3 months for NT-proBNP measurement [9, 10, 12]. The optimal monitoring interval during titration of treatment in NT-proBNP-guided therapy should be further investigated.
Stratified analysis for minimal informative monitoring interval of NT-proBNP
The minimal informative monitoring interval shortened along with the accumulation of risk represented by an increase of the AHEAD score. The present results indicate the need for tailored monitoring intervals for NT-proBNP levels in stable heart failure patients based on underlying risks. The difference in minimal informative intervals between patients with higher and lower ejection fractions was driven by a significantly smaller noise in the higher ejection fraction subgroup (1.89 ×10−1 ng2/L2 [95%CI: 1.65 ×10−1 to 2.03 ×10−1]) than in the lower ejection fraction subgroup (2.61 ×10−1 ng2/L2 [95%CI: 2.36 ×10−1 to 2.93 ×10−1]). This was inconsistent with the results from 2 previous studies, which suggested that variability in NT-proBNP was not influenced by the left ventricular ejection fraction [30, 31]. However, one of those studies only recruited subjects with an ejection fraction > 50% [31]. while the other mostly included patients with an ejection fraction < 40% [30]. The present study included both evenly and for the first time demonstrated that the intra-individual variability was smaller in patients with a preserved ejection fraction, compared with those with a reduced ejection fraction.
Study limitations
This study has several main limitations. First, this is a single center retrospective cohort study with a limited number of patients. Decisions regarding the schedule of NT-proBNP measurement, as well as alteration of medication regimen or readmission were left to the physicians’ discretion. The results of the present study should be externally validated. Second, the patients readmitted for heart failure within 6 months after the index admission, who might have benefitted the most from NT-proBNP measurement, were excluded. Finally, our model was based on the assumption that log (NT-proBNP) changes linearly in patients with a stable status of chronic heart failure. The linearity of its change remains to be further investigated.
Conclusions
Utilizing a statistical method taking into consideration the biological variability and measurement error of the NT-proBNP level, we determined that the overall minimal informative interval for monitoring NT-proBNP levels was 7.9 months (95%CI: 5.1–9.6) in the current population, while the intra-individual variability corresponded to a 61% increase from baseline. Of note, the minimal informative monitoring interval was shorter in patients with a worse predicted prognosis. These results emphasize the limitation of frequent measurement of NT-proBNP, the importance of detecting signs of heart failure exacerbation other than NT-proBNP, as well as the necessity of individualization in planning follow-up visits according to different risk profiles.
Availability of data and materials
The datasets generated and analyzed in the current study are not publicly available due to restrictions by the institutional research board.
Abbreviations
- NT-proBNP:
-
N-terminal pro-B-type natriuretic peptide
- IQR:
-
Interquartile range
- SD:
-
Standard deviation
- eGFR:
-
Estimated glomerular filtration rate
- CI:
-
Confidence interval
References
Bettencourt P, Azevedo A, Pimenta J, Frioes F, Ferreira S, Ferreira A. N-terminal-pro-brain natriuretic peptide predicts outcome after hospital discharge in heart failure patients. Circulation. 2004;110:2168–74.
Hartmann F, Packer M, Coats AJ, Fowler MB, Krum H, Mohacsi P, et al. Prognostic impact of plasma N-terminal pro-brain natriuretic peptide in severe chronic congestive heart failure: a substudy of the Carvedilol prospective randomized cumulative survival (COPERNICUS) trial. Circulation. 2004;110:1780–6.
Kang SH, Park JJ, Choi DJ, Yoon CH, Oh IY, Kang SM, et al. Prognostic value of NT-proBNP in heart failure with preserved versus reduced EF. Heart. 2015;101:1881–8.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776–803.
Mueller C, McDonald K, de Boer RA, Maisel A, Cleland JGF, Kozhuharov N, et al. Heart failure Association of the European Society of cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail. 2019;21:715–31.
Januzzi JL Jr, Rehman SU, Mohammed AA, Bhardwaj A, Barajas L, Barajas J, et al. Use of amino-terminal pro-B-type natriuretic peptide to guide outpatient therapy of patients with chronic left ventricular systolic dysfunction. J Am Coll Cardiol. 2011;58:1881–9.
Kaufmann BA, Goetschalckx K, Min SY, Maeder MT, Bucher U, Nietlispach F, et al. Improvement in left ventricular ejection fraction and reverse remodeling in elderly heart failure patients on intense NT-proBNP-guided therapy. Int J Cardiol. 2015;191:286–93.
Khan MS, Siddiqi TJ, Usman MS, Sreenivasan J, Fugar S, Riaz H, et al. Does natriuretic peptide monitoring improve outcomes in heart failure patients? A systematic review and meta-analysis. Int J Cardiol. 2018;263:80–7.
Felker GM, Anstrom KJ, Adams KF, Ezekowitz JA, Fiuzat M, Houston-Miller N, et al. Effect of natriuretic peptide-guided therapy on hospitalization or cardiovascular mortality in high-risk patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2017;318:713–20.
Eurlings LW, van Pol PE, Kok WE, van Wijk S, Lodewijks-van der Bolt C, Balk AH, et al. Management of chronic heart failure guided by individual N-terminal pro-B-type natriuretic peptide targets: results of the PRIMA (Can PRo-brain-natriuretic peptide guided therapy of chronic heart failure IMprove heart fAilure morbidity and mortality?) study. J Am Coll Cardiol. 2010;56:2090–100.
Stienen S, Salah K, Moons AH, Bakx AL, van Pol P, Kortz RAM, et al. NT-proBNP (N-terminal pro-B-type natriuretic peptide)-guided therapy in acute decompensated heart failure: PRIMA II randomized controlled trial (can NT-ProBNP-guided therapy during hospital admission for acute decompensated heart failure reduce mortality and readmissions?). Circulation. 2018;137:1671–83.
Pfisterer M, Buser P, Rickli H, Gutmann M, Erne P, Rickenbacher P, et al. BNP-guided vs symptom-guided heart failure therapy: the trial of intensified vs standard medical therapy in elderly patients with congestive heart failure (TIME-CHF) randomized trial. JAMA. 2009;301:383–92.
Schou M, Gustafsson F, Kjaer A, Hildebrandt PR. Long-term clinical variation of NT-proBNP in stable chronic heart failure patients. Eur Heart J. 2007;28:177–82.
Araujo JP, Azevedo A, Lourenco P, Rocha-Goncalves F, Ferreira A, Bettencourt P. Intraindividual variation of amino-terminal pro-B-type natriuretic peptide levels in patients with stable heart failure. Am J Cardiol. 2006;98:1248–50.
Bruins S, Fokkema MR, Romer JW, Dejongste MJ, van der Dijs FP, van den Ouweland JM, et al. High intraindividual variation of B-type natriuretic peptide (BNP) and amino-terminal proBNP in patients with stable chronic heart failure. Clin Chem. 2004;50:2052–8.
Wu AH, Smith A, Wieczorek S, Mather JF, Duncan B, White CM, et al. Biological variation for N-terminal pro- and B-type natriuretic peptides and implications for therapeutic monitoring of patients with congestive heart failure. Am J Cardiol. 2003;92:628–31.
Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham heart study subjects. Circulation. 1993;88:107–15.
Nishii M, Inomata T, Takehana H, Naruke T, Yanagisawa T, Moriguchi M, et al. Prognostic utility of B-type natriuretic peptide assessment in stable low-risk outpatients with nonischemic cardiomyopathy after decompensated heart failure. J Am Coll Cardiol. 2008;51:2329–35.
R Core Team. R: A Language and Environment for Statistical Computing. https://www.R-project.org. Accessed 2 Apr 2019.
Glasziou PP, Irwig L, Heritier S, Simes RJ, Tonkin A, Investigators LS. Monitoring cholesterol levels: measurement error or true change? Ann Intern Med. 2008;148:656–61.
Ohde S, McFadden E, Deshpande GA, Yokomichi H, Takahashi O, Fukui T, et al. Diabetes screening intervals based on risk stratification. BMC Endocr Disord. 2016;16:65.
Oke JL, Stevens RJ, Gaitskell K, Farmer AJ. Establishing an evidence base for frequency of monitoring glycated haemoglobin levels in patients with type 2 diabetes: projections of effectiveness from a regression model. Diabet Med. 2012;29:266–71.
Klersy C, d'Eril GV, Barassi A, Palladini G, Comelli M, Moratti R, et al. Advantages of the lognormal approach to determining reference change values for N-terminal propeptide B-type natriuretic peptide. Clin Chim Acta. 2012;413:544–7.
Raymond I, Groenning BA, Hildebrandt PR, Nilsson JC, Baumann M, Trawinski J, et al. The influence of age, sex and other variables on the plasma level of N-terminal pro brain natriuretic peptide in a large sample of the general population. Heart. 2003;89:745–51.
Krauser DG, Lloyd-Jones DM, Chae CU, Cameron R, Anwaruddin S, Baggish AL, et al. Effect of body mass index on natriuretic peptide levels in patients with acute congestive heart failure: a ProBNP investigation of dyspnea in the emergency department (PRIDE) substudy. Am Heart J. 2005;149:744–50.
Balion CM, Santaguida P, McKelvie R, Hill SA, McQueen MJ, Worster A, et al. Physiological, pathological, pharmacological, biochemical and hematological factors affecting BNP and NT-proBNP. Clin Biochem. 2008;41:231–9.
Carpenter J, Bithell J. Bootstrap confidence intervals: when, which, what? A practical guide for medical statisticians. Stat Med. 2000;19:1141–64.
Spinar J, Jarkovsky J, Spinarova L, Mebazaa A, Gayat E, Vitovec J, et al. AHEAD score–long-term risk classification in acute heart failure. Int J Cardiol. 2016;202:21–6.
Hox JJ. Multilevel analysis: techniques and applications. 2nd ed. New York: Routledge; 2010.
Frankenstein L, Remppis A, Frankenstein J, Hess G, Zdunek D, Slottje K, et al. Variability of N-terminal probrain natriuretic peptide in stable chronic heart failure and its relation to changes in clinical variables. Clin Chem. 2009;55:923–9.
Rosello-Lleti E, Calabuig JR, Morillas P, Cortes R, Martinez-Dolz L, Almenar L, et al. Variability of NT-proBNP and its relationship with inflammatory status in patients with stable essential hypertension: a 2-year follow-up study. PLoS One. 2012;7:e31189.
Acknowledgements
Parts of this work have been presented at the European Society of Cardiology Congress 2019, Paris, France. The authors thank the staff of the Cardiovascular Center, St. Luke’s International Hospital (Tokyo, Japan) for their continuous support and for providing quality patient care. The authors are also grateful to Ms. Naoko Tamagawa and Mr. Tsutomu Motoki from the Center for Information Systems, St. Luke’s International Hospital, for their excellent technical support.
Funding
No funding was related to the current study.
Author information
Authors and Affiliations
Contributions
ZD, TA, and SO designed the study. ZD and SO analyzed data. ZD, TA, SO, OT, and NK interpreted data. ZD and TA drafted the manuscript and SO, OT, and NK critically revised it. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The institutional research board of St. Luke’s International University approved the study (NO. 18-R070). All patients provided informed consent under an opt-out policy. Patients were able to opt out through telephone, mail, email, or direct visit. The opt-out policy was approved by the institutional research board, given the retrospective and observational nature of the study, where no additional invasive procedure or collection of any biological sample was required for the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Supplemental Table 1
. Estimates of the random-effects model for the overall stable heart failure population. Supplemental Fig. 1. Schematic of analytic period and follow-up NT-proBNP measurements.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Dai, Z., Asano, T., Takahashi, O. et al. The minimal informative monitoring interval of N-terminal pro-B-type natriuretic peptide in patients with stable heart failure. BMC Cardiovasc Disord 20, 262 (2020). https://doi.org/10.1186/s12872-020-01537-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-020-01537-7