Abstract
Background
To study the oxygen uptake efficiency and determine usefulness of submaximal parameters of oxygen uptake in systemic lupus erythematosus associated pulmonary arterial hypertension (SLE PAH) on performing a cardiopulmonary exercise test (CPET).
Methods
CPET was performed in 21 SLE PAH patients, equal number of idiopathic pulmonary arterial hypertension (IPAH) patients and controls. Peak VO2, anaerobic threshold (AT), oxygen uptake efficiency slope (OUES) and oxygen uptake efficiency plateau (OUEP) and other CPET parameters were examined. All subjects had pulmonary function test (PFT) at rest, which included FEV1, FVC, FEV1/FVC, DLCO measurements. Right heart catheterization (RHC) was also done in SLE PAH and IPAH patients. CPET parameters were compared with RHC parameters to determine potential correlations.
Results
Peak VO2, PETCO2 and peak O2 pulse were lower in SLE PAH than IPAH and controls with OUE being lower during all stages of exercise in SLE PAH. DLCO and FVC values were significantly lower in SLE PAH (p < 0.05). Peak O2 pulse and VO2@AT in SLE PAH and IPAH was low (p < 0.05) and significant difference between SLE PAH and IPAH was seen (p < 0.05). PVR correlated with the lowest VE/VCO2, O2 pulse, peak PETCO2 and OUE in SLE PAH patients (all p < 0.05).
Conclusions
SLE PAH patients have cardiopulmonary exercise limitation with reduced oxygen uptake efficiency. VO2@ at AT, peak O2 pulse and O2 pulse at AT were significantly reduced (p < 0.05). Key CPET parameters correlated with elevated pulmonary vascular resistance (PVR). Submaximal parameters of oxygen uptake are equally useful in SLE PAH.
Similar content being viewed by others
Background
Systemic lupus erythematosus (SLE) is an autoimmune disease with heterogenous clinical manifestations. Pulmonary hypertension is a rarer complication of SLE, occurring in less than 5% of patients [1]. Almost half of SLE patients have some kind of pulmonary involvement and about a quarter of them have cardiac involvement during the course of their disease [2]. Pulmonary hypertension is an indicator of advanced disease in itself regardless of cardiac, pulmonary or of vascular origins. In a country like China with a humongous population even a low estimated incidence of pulmonary arterial hypertension (PAH) in SLE has a huge implication.
The ERS/ESC guidelines endorse the use of exercise testing for the diagnosis and treatment of pulmonary hypertension [3] and it has also been suggested that CPET variables have important clinical application for patients with PAH [4]. Although CPET can indirectly reflect gas exchange during a given exercise test, gas exchange and exercise capacity are closely coupled to both cellular and cardio-pulmonary mechanisms. This is central to all current and cited recommendations [5]. CPET’s ability to reflect quite accurately the VO2 and associated parameters [6] and its ability to provide reproducible assessment of functional capacity and treatment efficacy in patients [7] contributes to its growing clinical utility. CPET has been suggested for use both in diagnostic evaluation (Level B, Class II a), prognostication (Level B, Class II b), and determining therapeutic efficiency (Level C, Class II b) [6]. Use of oxygen uptake efficiency (OUE) in a submaximal exercise test has already been widely studied in patients with exercise limitation due to cardio-pulmonary diseases [8,9,10,11].
Despite numerous studies in combined connective tissue diseases, CPET data for both maximal and submaximal parameters for SLE PAH are still lacking. Thus, the primary objective of the present study was to study the OUE and determine usefulness of submaximal parameters of oxygen uptake in SLE PAH on performing a CPET and to correlate CPET measurements with hemodynamic variables as measured by the RHC.
Methods
Subjects
This study retrospectively enrolled 21 patients with SLE-PAH, 21 controls and an equal number of age, BMI and functional class matched IPAH patients at Shanghai Pulmonary Hospital from November 2009 to October 2015. Shanghai pulmonary hospital is a tertiary level specialty pulmonary hospital, thus confirmatory SLE diagnosis and its management was done at other centers, patients were on follow ups at those centers and stable at the time of evaluation. The diagnosis of pulmonary hypertension was based on clinical and laboratory data that included RHC to fulfill the currently accepted diagnostic criteria [12] (NICE, 2013). PAH was defined as a mean pulmonary arterial pressure (mPAP) ≥25 mmHg and a pulmonary arterial wedge pressure (PAWP) ≤ 15 mmHg [13] and with a normal or reduced cardiac output (CO), without known triggering factors. Subjects were excluded from the study if they had any evidence of: right-to-left cardiac shunt, history of coexisting lung diseases, forced vital capacity in the first second to the full vital capacity ratio (FEV1/FVC) < 65%, have undergone pulmonary endarterectomy or were anemic.
Patient demographics, their pulmonary function, N-terminal natriuretic peptide type-B (NT-pro BNP), haemodynamics and CPET data were collected.
Cardiopulmonary exercise testing
CPET was performed on an electromagnetically braked cycle ergometer (VIASprint 150 P coupled to a Lab manager CPX cart, CareFusion, Jaeger crop, Hoechberg, Germany) using a breath-by-breath system to record gas exchange data over 10-s intervals. The protocol consisted of 3 min of rest, followed by 3 min of unloaded pedaling at 60 rpm, subsequently, a progressively increasing workload of 10 to 25 W/min to the maximum tolerance and finally 5 min of recovery. If patients developed fatigue, dyspnoea, chest tightness or any other discomfort during the CPET they were allowed to stop at anytime.
Measurements included workload, minute ventilation (VE), carbon dioxide output (VCO2), oxygen uptake (VO2), oxygen pulse (VO2/HR), heart rate (HR), heart rate reserve, breathing reserve (BR), respiratory exchange ratio (RER) and breathing frequency (BF). Anaerobic threshold (AT) which represents the beginning of anaerobic metabolism was determined by the V-slope method [14]. Minute ventilation/carbon dioxide output (VE/VCO2) slope was obtained by linear regression analysis of the relation between VE and VCO2. OUES was computed using the following equation: VO2 = ‘a’ log VE + ‘b’, where ‘a’ = OUES [15]. OUEP was at 90 s of the highest consecutive values for VO2 (mL/min)/ VE (L/min). Oxygen uptake efficiency at AT (OUE@ AT) was taken as the 60-s average of consecutive values at and immediately before the AT [16]. All predicted CPET values were calculated using accepted equations [17].
Resting pulmonary function measurements
All subjects underwent resting measurements of forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), diffusing capacity of carbon monoxide (DLCO), forced expiratory flow at 25% of forced vital capacity (FEF25), forced expiratory flow at 50% of forced vital capacity (FEF50), and forced expiratory flow at 75% of forced vital capacity (FEF75) using ATS/ERS criteria with results reported in absolute terms and % predicted values (e.g., FVC% and PEF50%), residual volume (RV) and total lung capacity (TLC) using established standards [18,19,20] and equipment (Jaeger Corp., Hoechberg, Germany). All predicted spirometry values were calculated using standardized and accepted equations for Chinese [21].
Right heart catheterization
All SLE PAH and IPAH patients underwent RHC with a true size thermodilution catheter (Edward 774) inserted via the left cubital fossa. Mean hemodynamic measurements included mPAP, PAWP, and mean right atrial pressure (mRAP). CO was obtained using the thermodilution method. Pulmonary vascular resistance (PVR) was calculated using standard formula: PVR = (mPAP-PAWP)/CO. Cardiac index (CI) was also calculated.
Statistical analysis
Data were analyzed using SPSS 20.0 (SPSS Inc.; Chicago, IL). Continuous variables are presented as mean ± SD, and categorical variables are presented as a percentage. Most PFT and CPET values are expressed in absolute terms and %pred. Unpaired Student t test was used to identify differences between groups and to compare between the three groups at each time period one way analysis of variance (ANOVA) was used along with post hoc analysis. Correlations between CPET parameters and hemodynamic variables were determined by Pearson’s correlation test. P value of < 0.05 was considered significant.
Results
Demographics and pulmonary function test
Gender, age and body mass index (BMI) were similar between SLE-PAH, IPAH and control group (Table 1). The FVC and FEV1 were lower in SLE-PAH group as compared to IPAH and controls. FEV1/FVC was not much different between the groups, (SLE-PAH 83.7 ± 6.4, IPAH 81.1 ± 8.1 and control group 85.9 ± 6.6). The DLCO and its %pre in SLE-PAH and IPAH was not only significantly lower than in controls, there was a significance between the two patient groups as well [SLE PAH 12.7 ± 3.4, IPAH 17.1 ± 5.0, and control 19.2 ± 3.9, p < 0.05], indicating altered gas exchange efficiency. The decrease in TLC was not very remarkable between the groups; SLE-PAH patients exhibited more reduced values (SLE-PAH 3.8 ± 0.9, IPAH 4.64 ± 0.72, and controls 4.6 ± 0.5). Both the observed and %pred FVC was markedly reduced compared to the controls and a significant difference was noted between SLE PAH and IPAH as well (p < 0.05). (Table 1).
Cardiopulmonary exercise test measurements
Peak work load and the % pred peak work load in SLE-PAH were markedly reduced as compared to IPAH and controls. The peak VO2 and % pred peak VO2 in SLE-PAH was 766 ± 258 (55 ± 18), IPAH 903 ± 207 (47.95 ± 22.17) and control 1335 ± 232 (95 ± 40). The peak HR in SLE PAH was 147.3 ± 19.7, IPAH 144.2 ± 26.1 and control 161.7 ± 18.1. The VE/VCO2 slope in both patient groups in this study was significantly higher than in controls. The peak O2 pulse was also reduced in both patient groups, i.e. SLE PAH 5.2 ± 1.3, IPAH 6.4 ± 1.6, p < 0.05 VS 8.2 ± 1.1 in the controls and a statistically significant difference was seen between SLE PAH and IPAH as well (p < 0.05). The peak VE was also reduced in both SLE PAH and IPAH, p < 0.05. Peak PeTCO2 were reduced in both SLE-PAH and IPAH. VO2 AT and its %pred was also markedly different in the three groups, i.e. SLE-PAH 554 ± 182 (53.9 ± 16.2), IPAH 678.2 ± 156 (57.3 ± 16.4) and control group 896 ± 171 (85.9 ± 30.1). Although the lowest VE/VCO2 and its %pred were almost comparable between SLE-PAH and IPAH and were significantly deranged when compared to the controls, it was nevertheless, correlated well with the PVR in both patient groups. The OUES and its %pred were also likewise deranged in SLE-PAH and IPAH as compared to the controls. The OUEP and %pred in SLE-PAH and IPAH was markedly reduced than in controls. The peak VO2 particularly correlated to OUES and OUEP in both SLE PAH and IPAH (p < 0.005). SLE PAH, OUES with peak VO2, r 0.78, p < 0.001, while in IPAH r − 0.62, p < 0.005 and SLE PAH, peak VO2 with OUEP, r 0.69, p 0.001, while in IPAH r 0.42, p 0.06). Significant correlation of OUEP % pred with peak VO2 was seen in SLE PAH group, i.e. SLE PAH r 0.68, p 0.001 and in IPAH r 0.24, p 0.276 respectively. OUEP % pred with peak VO2% pred in the SLE group was, r 0.54, p 0.01 while in IPAH was r 0.41, p 0.06. Ventilation at AT and VE/VCO2 slope were also significantly lower in SLE-PAH than in IPAH and controls (both P < 0.05, Table 1).
Although HR increased as exercise intensity increased in all three cohorts the increase was not optimal in SLE-PAH and IPAH as compared to control subjects indicating an impaired chronotropic reserve in such patients (Fig. 1b). The peak PETCO2 was statistically significant between control and SLE PAH and IPAH and correlated with PVR in both patient groups. Correlations between key parameters are listed in Tables 3 and 4.
Right heart catheterization
Right heart hemodynamics was compared between SLE-PAH and IPAH patients. The mPAP, RAP, PAWP and PVR were all lower in SLE PAH than in IPAH (p < 0.05). The CO in both groups was almost comparable. However, though the CI was lower in IPAH patients on an average it was not statistically significant (Table 2).
On comparing the correlation of PVR with VE/VCO2 slope % pred and lowest VE/VCO2% pred in the two disease groups significant correlations were noted (Tables 3 and 4).
The NYHA functional class and CO were comparable between SLE PAH and IPAH but their mPAP, RAP, PVR and PAWP were different (Table 2, Fig. 1a). Although SLE PAH patients had lower mPAP, RAP, PVR and PAWP values there was no statistical difference with NTproBNP levels between SLE PAH and IPAH patients.
Discussion
The clinical features of heart failure typically seen in the general population are less likely to be evident at presentation in patients with lupus [22]. PAH is the third leading cause of death in Chinese SLE patients [23] and the Chinese SLE treatment and research group (CSTAR) estimates the prevalence of PAH in SLE in china to be 3.8% with a very skewed female to male ratio (F:M = 10.1) [24]. The incidence was found to be 12% for CTD associated PAH and 25% for IPAH in our institution (shanghai pulmonary hospital, unpublished data). Early identification and adequate treatment is particularly important as SLE-PAH patients by comparison have poorer prognosis [25]. It has also been demonstrated that PAH is an independent risk factor for mortality in SLE [26] and predisposes to increased cardiovascular events despite late onset or mild lupus [27]. CPET has proven very useful in noninvasively evaluating functional limitations in other patient groups. Thus, we sought to evaluate characteristics of CPET in SLE PAH.
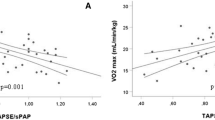
Peak VO2 is reduced in patients with higher pulmonary vascular resistance and is highly correlated with the amount of functional pulmonary vascular bed [28]. When the cardiac compensation is not adequate both the peak VO2 and the O2 pulse are reduced, both of which were reduced in SLE PAH patients (Fig. 1c). After Baba et al. [15]proposed the usefulness of OUES, other parameters like OUEP %pred and lowest VE/VCO2% pred have since also been used as reliable indicators of exercise capacity in PAH patients [29,30,31,32] in addition to the peak VO2. We also noted peak VO2 to be particularly correlated to OUES and OUEP in both SLE PAH and IPAH (p < 0.005). OUE can be altered by CO, difference in pulmonary to systemic arterial oxygen tension, effectiveness of the respiratory membrane and the blood pH. The OUE is markedly impaired during all phases in SLE PAH patients and the peak is much more subtle than in IPAH or controls, which reflects the increased metabolic demand during the AT and peak not being met in such patients (Fig. 1d). This concurs with the report by Tan et al. [10] who reported similar results for IPAH patients. Despite cardiopulmonary reserve being adequate at rest, the OUE in SLE PAH is abnormal, resulting perhaps as a consequence of the increased pulmonary vascular resistance. We had reported similar findings in patients with pulmonary embolism [33].
Patients with a peakVO2 < 10 ml/kg per min are generally accepted to have a poor prognosis. A majority of our subjects had peak VO2 levels between 10 and 18 ml/kg per min. The VE/VCO2 slope in both the patient groups in this study was significantly higher than in controls. Corra et al. postulated that in such patients the VE/VCO2 slope may represent a proper descriptor of the heterogeneity of haemodynamics and neuro-humoral adaptations to exercise and disorders of ventilatory reflex control [34]. Based on their cut off values our patients in class II/III have to be classified as being at moderate risk for cardiac events.
SLE PAH patients had reduced FVC, FEV1, MVV and TLC. It has been suggested that a low %FVC also affects survival in PAH [35]. Though the extent of the disease could not be accurately quantified owing to lack of CT findings, based on the observed FVC in SLE PAH patients, suggests they have intermediate interstitial disease [36].
The peak VO2 was lower, the VE/VCO2 slope was higher and the PETCO2 was lower in SLE PAH patients, which is consistent with earlier reports, but what is not consistent is the magnitude of change in CPET variables was greater in SLE PAH rather than IPAH [6] in the current study. A reduced peak VO2, an increased VE/VCO2 slope with reduced ventilatory efficiency i.e. low peak/end exercise PETCO2 are clearly indicative of impaired pulmonary function and are key parameters of outcomes in PAH patients. Moreover peak PETCO2 has been reported to be an independent predictor of increased PVR in PAH patients [37] with similar findings being reported previously [38].
The increased pulmonary vascular resistance perhaps alters cardiac output (i.e. lower VO2@AT and lower peak O2 pulse) and pulmonary blood flow which in turn leads to reduced oxygen uptake as seen in our subjects, indicating that SLE PAH patients have markedly altered exercise capacity owing to cardiac and pulmonary factors.
The presence of pulmonary hypertension further enhances the decrease of DLCO indicating poor gas exchange even at rest [39,40,41,42]. IPAH patients with reduced DLCO were reported to have worse exercise performance despite having similar hemodynamic profiles [43]. This corroborates with our findings as well, furthermore the altered DLCO in SLE PAH was more severe in our patients. The reduced DLCO perhaps occurs as a combination of alterations in the respiratory membrane along with alteration in small pulmonary vessels [29]. It seems plausible that the reduced gas exchange efficiency in SLE PAH results more from pulmonary consequences as their mPAP, PVR and PAWP were lower than IPAH. PVR correlated better with other CPET parameters rather than RER in the present study (Tables 3 and 4), which is different from an earlier report where RER correlated better with PVR in IPAH [35]. Since the vascular resistance in SLE PAH is not as high as in IPAH, implying that SLE PAH patients perhaps develop more gradual cardiac consequences as compared to IPAH but faster and enhanced pulmonary/pulmonary vascular ones. As PVR is also related to lung volumes, being lowest at the functional residual capacity, the interstitial/restrictive pathology in the lungs due to SLE PAH in combination with small pulmonary vessel alterations perhaps contributes more towards the altered gas exchange efficiency. A lower PVR and better CI are an evidence of a bit better right heart hemodynamics in SLE PAH as compared to IPAH. Whether the slightly higher NTproBNP seen in SLE is a reflection of a difference in right atrial modification between the two disease groups or just an aberrant result needs to be explored further. Although beyond the scope of the current study it would be interesting to find out more about dynamic changes of the PVR during exertion in an optimally performed CPET.
Increased VE/VCO2 has been correlated with decreased cardiac output, elevated pulmonary arterial pressures, decreased alveolar capillary membrane conductance and diminished heart rate variability [43,44,45], we also noted correlations between PVR, VE/VCO2 SLOPE % pred and lowest VE/VCO2% in the two disease groups.
Though the hemodynamics data and NTproBNP levels were a bit higher than our subjects in earlier reports from China using CTD/SLE cohorts [46, 47], the demographics were comparable (Table 5). They used the 6MWD which is not adequate to judge activity levels and treatment response and thus we could not compare their results with ours for consistency as well. SLE PAH patients have distinct trends when performing a CPET. We agree with using established parameters like the peak VO2, PETCO2 and O2 pulse, but also recommend using the OUE (OUES, OUEP), as it is reliable when derived even from submaximal exercise.
Conclusions
In conclusion, SLE PAH patients have gas exchange impairment that is compounded by an inadequate cardiac compensation. This fact adds to the already known premise that SLE PAH patients have faster disease progression and worse prognosis. Since ours is a tertiary level referral center observer bias cannot be ruled out. This is a single center retrospective study with a small cohort of patients. The lack of imaging data limits our understanding regarding potential cardiovascular modifications and pulmonary processes occurring with the concurrent disease process. Although there is sufficient knowledge that incremental exercise tests in general are better than submaximal constant workload tests with regards to the intensity levels, we however note that in a study like ours employing a constant workload test would reflect more uniformly their exercise capacity. Despite this we have examined relevant parameters of CPET on a per kg body weight format where applicable to minimize bias. We also did not categorically calculate the SLE disease activity index or the medications they were already on at the time of the test. Nevertheless, we believe the results we have presented have future reference value. Further validations of these results are warranted to translate into clinical utility.
Abbreviations
- CI:
-
Cardiac index
- CO:
-
Cardiac output
- CPET:
-
Cardiopulmonary exercise test
- IPAH:
-
Idiopathic pulmonary arterial hypertension
- mPAP:
-
Mean pulmonary artery pressure
- mRAP:
-
Mean right atrial pressure
- NT-pro BNP:
-
N-terminal natriuretic peptide type-B
- OUE:
-
Oxygen uptake efficiency
- OUEP:
-
Oxygen uptake efficiency plateau
- OUES:
-
Oxygen uptake efficiency slope
- PAWP:
-
Pulmonary arterial wedge pressure
- Peak VO2 :
-
Peak oxygen uptake
- PVR:
-
Pulmonary vascular resistance
- RER:
-
Respiratory exchange ratio
- RHC:
-
Right heart catheterization
- SLE PAH:
-
Systemic lupus erythematosus associated pulmonary arterial hypertension
- VCO2 :
-
Carbon dioxide output
- VE :
-
Minute ventilation
- VO2 :
-
Oxygen uptake
- VO2/HR:
-
Oxygen pulse
References
Longo Dan, Fauci A, Kasper D, Hauser S, Jameson J, Loscalzo J. Harrison’s principles of internal medicine 18th edition, Chapter 319, page 2731, 2012, McGrawHill companies, USA.
Kumar P, Clark M. Kumar & Clark Clinical Medicine. 6th ed. India: Elsevier Saunders Ltd.; 2005. p. 575.
Galiè N, Humbert M, Vachiery J, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2015;46(4):903–75. PMID: 26318161
Guazzi M, Adams V, Conraads V, et al. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation. 2012;126:2261–74. PMID: 22952317
Babu A, Arena R, Myers J, Padmakumar R, Maiya A, Cahalin L, et al. Exercise intolerance in pulmonary hypertension: mechanism, evaluation and clinical implications. Expert Rev Respir Med. 2016;10:979–90. PMID: 27192047
Pinkstaff S, Burger C, Daugherty J, Bond S, Arena R. Cardiopulmonary exercise testing in patients with pulmonary hypertension: clinical recommendations based on a review of the evidence. Expert Rev Respir Med. 2016;10(3):279–95. PMID: 26789612
Wensel R. Assessment of survival in patients with primary pulmonary hypertension: importance of cardiopulmonary exercise testing. Circulation. 2002;106:319–24. PMID: 12119247
Van Laethem C, Van De Veire N, De SJ, et al. Prospective evaluation of the oxygen uptake efficiency slope as a submaximal predictor of peak oxygen uptake in aged patients with ischemic heart disease. Am Heart J. 2006;152:297–315. PMID: 16875913
Defoor J, Schepers D, Reybrouck T. Oxygen uptake efficiency slope in coronary artery disease: clinical use and response to training. Int J Sports Med. 2006;27:730–7. PMID: 16944401
Tan X, Yang W, Guo J. Usefulness of decrease in oxygen uptake efficiency to identify gas exchange abnormality in patients with idiopathic pulmonary arterial hypertension. PLoSOne. 2014;9(6):e98889. PMID: 24905576
Shi X, Guo J, Gong S, Sapkota R, et al. Oxygen uptake is more efficient in idiopathic pulmonary arterial hypertension than in chronic thromboembolic pulmonary hypertension. Respirology. 2016;21(1):149–56. PMID: 26390972
Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25Suppl):D34–41. PMID: 24355639
Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62:D42–50. PMID: 24355641
Bentley DJ, McNaughton LR. Comparison of W (peak), VO2(peak) and the ventilation threshold from two different incremental exercise tests: relationship to endurance performance. J Sci Med Sport. 2003;6:422–35. PMID: 14723392
Baba R, Nagashima M, Goto M, et al. Oxygen uptake efficiency slope: a new index of cardiorespiratory functional reserve derived from the relation between oxygen uptake and minute ventilation during incremental exercise. J Am Coll Cardiol. 1996;28:1567–72. PMID: 8917273
Sun XG, Hansen JE, Stringer WW. Oxygen uptake efficiency plateau (OUEP): physiology and reference value. Eur J Appl Physiol. 2012;112:919–28. PMID: 21695524
Valois FM, Nery LE, Ramos RP, et al. Contrasting cardiopulmonary responses to incremental exercise in patients with schistosomiasis-associated and idiopathic pulmonary arterial hypertension with similar resting hemodynamic impairment. PLoS One. 2014;9(2):e87699. PMID: 24498356
Wasserman K, Hansen JE, Sue DY, et al. Principles of exercise testing and interpretation. 5th ed. Philadelphia: Lippincott, Williams & Wilkins; 2011.
MacIntyre N, Crapo RO, Viegi G, et al. Standardization of the single-breath determination of carbon monoxide uptake in the lung. ERJ. 2005;26(4):720–35. PMID: 16204605
Miller MR, Hankinson J, Brusasco V, et al. Standardization of spirometry. ERJ. 2005;26(2):319–38. PMID: 16055882
Zheng J, Zhong N. Normative values of pulmonary function testing in Chinese adults. Chin Med J. 2002;115:50–4. PMID: 11930658
Davis JM 3rd, Roger VL, Crowson CS, et al. The presentation and outcome of heart failure in patients with rheumatoid arthritis differs from that in the general population. Arthritis Rheum. 2008;58(9):2603–11. PMID: 18759286
Hao Y-J, Jiang X, Wei Z, Yong W, et al. Connective tissue disease associated pulmonary arterial hypertension in chinese patients. Eur Respir J. 2014;44:963–72. 24791829
Li M, Wang Q, Zhao J, et al. Chinese SLE treatment and research group (CSTAR) registry: II. Prevalence and risk factors of pulmonary arterial hypertension in Chinese patients with systemic lupus erythematosus. Lupus. 2014;23(10):1085–91. PMID: 24651670
Johnson SR, Granton JT, et al. Pulmonary hypertension in systemic sclerosis and systemic lupus erythematosus. Eur Respir Rev. 2011;20:277–86. PMID: 22130821
Min HK, Lee JH, Jung SM, et al. Pulmonary hypertension in systemic lupus erythematosus: an independent predictor of patient survival. Korean J Intern Med. 2015;30(2):232–41. PMID: 25750566
Bartels CM, Buhr KA, Goldberg JW, et al. Mortality and cardiovascular burden of systemic lupus erythematosus in a US population-based cohort. J Rheumatol. 2014;41(4):680–7. PMID: 24532834
Guazzi M, Myers J, Arena R. Cardiopulmonary exercise testing in the clinical and prognostic assessment of diastolic heart failure. J Am Coll Cardiol. 2005;46:1883–90. PMID: 16286176
Sun XG, Hansen JE, Oudiz RJ, et al. Exercise pathophysiology in patients with primary pulmonary hypertension. Circulation. 2001;104:429–35. PMID: 11468205
Sun XG, Hansen JE, Garatachea N, et al. Ventilatory efficiency during exercise in healthy subjects. Am J Respir Crit Care Med. 2002;166:1443–8. PMID: 12450934
Shi X, Liu J, Guo J, Yang W, Liu H, Huang D, Wu C, Gong S, Zhao Q, He J, Wang L. Correlation between submaximal exercise measurements and peak oxygen uptake in patients with pulmonary arterial hypertension. Zhonghua Yi Xue Za Zhi (Chinese medical journal). 2014;94(32):2490–4. PMID: 25410918 (Article in chinese)
Jian G, Shujuan C, Pudasaini B, et al. Oxygen uptake efficiency slope, an objective submaximal parameter in evaluating exercise capacity in pulmonary thromboembolism. Am J Med Sci. 2016;351(5):485–91. PMID: 27140707
Fei Y, Shi X, Gan F, Li X, et al. Death causes and pathogens analysis of systemic lupus erythematosus during the past 26 years. Clin Rheumatol. 2014;33(1):57–63. PMID: 24046218
Corra U, Mezzani A, Bossimini E, Scapellato F, Imparato A, Gianuzzi P. Ventilatory response to exercise improves risk stratification in patients with chronic heart failure and intermediate functional capacity. Am Heart J. 2002;143:418–26.
Suzuki A, Taniguchi H, Watanab N, et al. Significance of pulmonary arterial pressure as a prognostic indicator in lung-dominant connective tissue disease. PLoS One. 2014;9(9):e108339. https://doi.org/10.1371/journal.pone.0108339.
Goh N, Desai SR, Veeraraghavan S, et al. Interstitial lung disease in systemic sclerosis: a simple staging system. Am J Respir Crit Care Med. 2008;177:1248–54.
Yuan P, Chen TX, Pudasaini B, et al. Sex-specific cardiopulmonary exercise testing indices related to hemodynamics in idiopathic pulmonary arterial hypertension. Ther Adv Respir Dis. 2017;11(3):135–45. PMID: 28043202
Babu AS, Myers J, Arena R, et al. Evaluating exercise capacity in patients with pulmonary arterial hypertension. Exp Rev Cardiovasc Ther. 2013;11:729–37. PMID: 23750682
Boros PW, Enright PL, Quanjer PH, Borsboom GJ, Wesolowski SP, Hyatt RE. Impaired lung compliance and DLCO but no restrictive ventilatory defect in sarcoidosis. Eur Respir J. 2010;36(6):1315–22. PMID: 20378598
Behr J, Ryu JH. Pulmonary hypertension in interstitial lung disease. Eur Respir J. 2008;31:1357–67. PMID: 18515559
Jing ZC, Xu XQ, Badesch DB, et al. Pulmonary function testing in patients with pulmonary arterial hypertension. Respir Med. 2009;103:1136–42. PMID: 19403296
Trip P, Nossent EJ, de Man FS, et al. Severely reduced diffusion capacity in idiopathic pulmonary arterial hypertension: patient characteristics and treatment responses. Eur Respir J. 2013;42:1575–85. PMID: 23949959
Reindl I, Werneck KD, Opitz C. Impaired ventilatory efficiency in chronic heart failure: possible role of pulmonary vasoconstriction. Am Heart J. 1998;136:778–85. PMID: 9812071
Mortara A, La Rovere MT, Pinna GD. Arterial baroreflex modulation of heart rate in chronic heart failure: clinical and hemodynamic correlates and prognostic implications. Circulation. 1997;96:3450–8. PMID: 9396441
Ponikowski P, Anker SD, Chua TP, et al. Depressed heart rate variability as an independent predictor of death in chronic congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1997;79:1645–50. PMID: 9202356
Zhang R, Dai LZ, Xie WP, et al. Survival of Chinese patients with pulmonary arterial hypertension in the modern treatment era. Chest. 2011;140:301–9. PMID: 21330386
Zeng X, Huang C, Li M, Liu Y, et al. Baseline characteristics and risk factors of pulmonary arterial hypertension in systemic lupus erythematosus patients. Med (LWW, Baltimore). 2016;95(10):e2761. PMID: 26962774
Acknowledgements
All authors wish to thank the technicians at the department of pulmonary function at shanghai pulmonary hospital.
Funding
This work was supported by (1) Science and Technology Commission of Shanghai Municipality’s project No.16ZR1429000, and (2) National Health and Family Planning Commission of the People’s Republic of China, Medical and Health Science Technology Development Research Center’s project no. W2015RNA09B.
Availability of data and materials
The data presented in the manuscript is self-explanatory any other authors seeking to use any part of the data can get in touch with the corresponding author.
Author information
Authors and Affiliations
Contributions
LJM contributed to outlining the concept of the study and oversaw the entire process of obtaining data & preparing the manuscript and is the corresponding author. BP, GLY & CY contributed to obtaining the data, performing the CPET, data processing and drafting the manuscript and therefore are first author and first co-authors respectively. JG, PY, WLY made substantial contributions design and with the PFT reports. SGG performed the RHC and helped with its data analysis. TXC, XW contributed to performing CPET while RZ, LW, QHZ and RJ contributed by revising the manuscript critically for important intellectual content. All authors have given approval for the final version to be published and have agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical approval to conduct this study was obtained from Shanghai pulmonary hospital’s ethical board and all participants gave written informed consent to participate in this study. The study was performed in accordance with the declaration of Helsinki.
Consent for publication
All authors had access to all the data used during the course of preparing this manuscript and all authors approved the manuscript prior to sending out for publication.
Competing interests
We have read BioMed Central’s guidance on competing interests and declare that none of the authors have any competing interests-financial or otherwise.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Pudasaini, B., Yang, Gl., Yang, C. et al. Characteristics of exercise capacity in female systemic lupus erythematosus associated pulmonary arterial hypertension patients. BMC Cardiovasc Disord 18, 56 (2018). https://doi.org/10.1186/s12872-018-0783-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-018-0783-7