Abstract
Background
Plaque shifting is a serious complication of endovascular treatment (EVT) for aortoiliac bifurcation lesions. It is challenging to predict the occurrence of unfavorable plaque shifting correctly.
Case presentation
We report the case of an 88-year-old Japanese woman who experienced constant pain at rest in her left leg. The ankle-brachial pressure index of her left leg was 0.57. Computed tomography (CT) angiography revealed severe stenosis of the left common iliac artery (CIA) and total occlusion of the left external iliac artery (EIA). We diagnosed the patient with acute exacerbation of a chronic limb ischemia and administered endovascular treatment (EVT) to treat the left CIA and EIA. The results of initial angiography agreed with those of CT angiography. After placing a self-expandable stent for the left CIA lesion, significant unfavorable plaque shifting occurred. From a comparison between pre- and post-stenting angiography, we realized that the plaque protrusion into the terminal aorta had formed a “pseudo aortoiliac bifurcation” that was situated more proximally compared to the true bifurcation. We had incorrectly assessed the height of the aortoiliac bifurcation and exact plaque position and had underestimated the risk of plaque shifting because of this misunderstanding. The patient ultimately developed fatal cholesterol embolization after EVT.
Conclusions
Plaque protrusion into the terminal aorta can form a “pseudo aortoiliac bifurcation”, causing the wrong estimation of the height of the aortoiliac bifurcation; “angiographically”, the highest point is not always the true bifurcation. Careful assessment of initial angiography to detect the true aortoiliac bifurcation and exact plaque position is essential to avoid unfavorable plaque shifting.
Similar content being viewed by others
Background
Endovascular treatment (EVT) at the level of the aortic bifurcation has been widely performed in recent years. The EVT strategy for aortoiliac bifurcation lesions has been discussed in the literature [1,2,3]. The most important concern with regard to these lesions is plaque shifting or embolization of the contralateral vessel. To avoid these serious complications, the kissing balloon technique, in which balloons are simultaneously positioned across both limbs of the aortic bifurcation and inflated in unison, has been reported to be effective [2]. Later as stents became widely used, the kissing stent technique was adapted instead of the kissing balloon technique, especially in the case of dissection, thrombosis, or significant residual stenosis [3]. However, it is still challenging to predict the occurrence of unfavorable plaque shifting correctly. Here, we report a case of significant unfavorable plaque shifting after stenting for an aortoiliac bifurcation lesion, caused by a “pseudo aortoiliac bifurcation” formed by plaque protrusion into the terminal aorta.
Case presentation
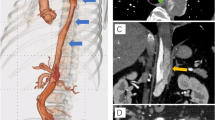
An 88-year-old Japanese woman with bacterial pneumonia was admitted to our hospital. Her medical history included angina pectoris and nontuberculous mycobacterial pulmonary infection. She had no history of atrial fibrillation. She occasionally felt pain at rest in her left leg during hospitalization. Pneumonia resolved with antibiotic therapy. On the 23rd day of hospitalization, she complained of constant pain at rest and cyanosis in the left leg. Two days later, the pain and skin color worsened. Her body temperature was 36.6 °C; blood pressure, 150/70 mmHg; regular pulse rate, 80 beats/min; and oxygen saturation, 93% (without oxygen administration). The ankle-brachial pressure index of the left leg was 0.57. Myogenic enzymes and lactic acid were not elevated in laboratory findings, and the left lower limb had not become necrosed. Computed tomography (CT) angiography revealed severe stenosis of the left common iliac artery (CIA) and total occlusion of the left external iliac artery (EIA) (Fig. 1a). As for the CIA lesion, the plaque seemed to exist at the ostium of the CIA (Fig. 1b-d). The arteries below the knee were also severely stenosed or occluded. We made a diagnosis of acute exacerbation of chronic limb ischemia and considered that the possibility of acute embolization was relatively low. We discussed the treatment strategy, including optimal medical therapy or surgical bypass grafts. Considering the patient’s age, severe symptoms, and general condition, we thought the endovascular approach would be more suitable. We administered EVT to treat the left CIA and EIA lesion, in addition to the medical treatment comprising heparin, aspirin, and alprostadil. The results of initial angiography agreed with those of CT angiography (Fig. 2), and we planned to place stents for both CIA and EIA lesions. Since the left common femoral artery (CFA) had moderate to severe stenosis and the crossover approach via the right CFA seemed to be difficult because of the left CIA lesion, we inserted a 6-Fr Sheathless PV® guiding catheter (Asahi Intecc, Japan) into the terminal aorta via the left brachial artery. We attempted antegrade wiring using a 0.018-in. wire with intravascular ultrasound (IVUS), changing wires several times. We finally passed a 0.014-in. wire into the left CFA. We performed pre-dilatation with a 4-mm balloon for both CIA and EIA lesions, and thrombus aspiration for the EIA lesion using a 6-Fr straight guiding catheter but no thrombus was aspirated. Subsequently, angiography showed good antegrade blood flow (Fig. 3). To simplify the adjustment of the stent deployment position, especially the stent proximal edge, we switched to the left CFA approach; we re-punctured the left CFA and passed the wire into the aorta. IVUS in the EIA lesion showed that the wire passed through the true lumen and the lesion consisted chiefly of mixed plaque with no calcification (Fig. 4). We did not perform IVUS for the CIA lesion. We first performed single-stenting using a self-expandable stent, an Epic® stent (120 × 40 mm) (Boston Scientific, USA), for the left CIA lesion, and then placed two other Epic® stents (90 × 100 mm and 80 × 40 mm, respectively) at the EIA lesion. After post-dilatation with a 5-mm balloon for each lesion, angiography showed significant plaque shifting to the right CIA (Fig. 5a, Additional file 1: Video S1). Moreover, the stent in the left CIA completely crossed over the contralateral CIA (Fig. 5b, Additional file 1: Video S1). We inserted a 6-Fr sheath via the right CFA and performed an IVUS for the shifted plaque. A large mobile plaque with a high risk of distal embolization was observed. We considered that it would be challenging to implant an additional stent in the right CIA to cover the plaque. Therefore, we performed long balloon inflation with an 8-mm balloon to compress and stabilize the shifted plaque. The final angiography showed good blood flow in both lower limbs with no evidence of distal embolization. Since there was no flow limitation or pressure gradient across the shifted plaque, we completed the procedure. On the day after EVT, the patient developed livedo reticularis on both lower limbs, with a high inflammatory response and worsening renal function. Although eosinophilia was not observed, we considered that the cause of death was cholesterol embolization based on this clinical course. Eventually, the patient died of multiple organ failure 3 days after EVT despite treatment.
Additional file 1: Video S1. Angiography after stent placement showed significant plaque shifting to right common iliac artery (arrows). (MP4 2203 kb)
Discussion
We present a case of significant plaque shifting after stenting in an aortoiliac bifurcation lesion. Because of the “pseudo aortoiliac bifurcation” formed by plaque protrusion into the terminal aorta, we incorrectly assessed the true aortoiliac bifurcation and exact plaque position and underestimated the risk of plaque shifting. To our knowledge, this is the first report of this phenomenon.
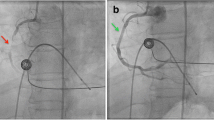
In the present case, we initially thought that “angiographically”, the highest point was the aortoiliac bifurcation and the plaque existed only in the left CIA (Fig. 6). We planned to perform stenting at the ostium of the left CIA with minimum protrusion into the aorta without obstructing the entry to the contralateral CIA. However, post-stenting angiography showed that the height of the aortoiliac bifurcation had changed, moving more distally (Fig. 7). From a careful comparison between the pre- and post-stenting angiography, we realized that we had incorrectly assessed the aortoiliac bifurcation and exact plaque position; a large plaque had protruded into the terminal aorta and formed the “pseudo aortoiliac bifurcation” (Fig. 8). The true aortoiliac bifurcation existed more distally than the “angiographically” highest point (Fig. 9). We underestimated the risk of plaque shifting because of this misunderstanding.
Our initial impression regarding the height of aortoiliac bifurcation and plaque position. We thought that “angiographically”, the highest point was the aortoiliac bifurcation and the plaque existed only in the left CIA. (left side: pre-stenting angiography, right side: post-stenting angiography, white dash line: the height of the aortoiliac bifurcation according to our initial impression, red dash line: the vessel wall line of aorta and bilateral iliac artery according to our initial impression, white line: plaque position according to our initial impression)
Comparison between pre-stenting and post-stenting angiography. Careful comparison showed that the height of the aortoiliac bifurcation had changed as against our initial impression; the height of the aortoiliac bifurcation moved more distally after stenting. (left side: pre-stenting angiography, right side: post-stenting angiography, white dash line: the height of the aortoiliac bifurcation according to our initial impression, red dash line: the height of the true aortoiliac bifurcation revealed by the post-stenting angiography)
On a retrospective review of the initial angiography, the vessel wall of the “pseudo aortoiliac bifurcation” was slightly unnatural. If we had assessed the initial angiography more carefully and noticed the plaque protrusion and the “pseudo aortoiliac bifurcation” beforehand, we would have performed the kissing balloon technique or kissing stent technique if dissection or residual stenosis had occurred. However, our initial evaluation that the plaque had not protruded into the aortoiliac bifurcation was wrong, leading to the single-stent strategy. We should have realized that the plaque could protrude into the terminal aorta and form the “pseudo aortoiliac bifurcation”, because “angiographically”, the highest point is not always the true aortoiliac bifurcation. Regarding the bailout method for the plaque shifting, there are no definitive methods for this kind of situation. In fact, we did not discover a better way to manage this complication. Thus, it is important to prevent unfavorable plaque shifting as much as possible.
Conclusions
We described a case of significant plaque shifting due to a “pseudo aortoiliac bifurcation” formed by plaque protrusion into the terminal aorta. Careful assessment of initial angiography is essential for detecting the true aortoiliac bifurcation and exact plaque position in order to prevent unfavorable plaque shifting.
Abbreviations
- CFA:
-
Common femoral artery
- CIA:
-
Common iliac artery
- CT:
-
Computed tomography
- EIA:
-
External iliac artery
- EVT:
-
Endovascular treatment
- IVUS:
-
Intravascular ultrasound
References
Tegtmeyer CJ, Wellons HA, Thompson RN. Balloon dilation of the abdominal aorta. JAMA. 1980;244:2636–7.
Tegtmeyer CJ, Kellum CD, Kron IL, Mentzer RM Jr. Percutaneous transluminal angioplasty in the region of the aortic bifurcation. The two-balloon technique with results and long-term follow-up study. Radiology. 1985;157:661–5.
Mendelsohn F, Santos RM, Crowley JJ, Lederman RJ, Cobb FR, Phillips HR, et al. Kissing stents in the aortic bifurcation. Am Heart J. 1998;136:600–5.
Acknowledgments
None.
Funding
None.
Availability of data and materials
All data is available in the manuscript.
Author information
Authors and Affiliations
Contributions
YK: Managing the patient, writing the manuscript, and revising the manuscript. TK: Writing, correcting, and revising the manuscript. DN: Management the patient and revising the manuscript. KZ: Revising the manuscript and the figures. SM: Revising the manuscript and the figures. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent for publication
Written informed consent was obtained from the patient’s son for publication of this case report and any accompanying images and videos. A copy of the written consent is available for review by the Editor of this journal.
Competing interests
All authors have no financial interests to disclose and no conflicts of interest to declare.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Kadoya, Y., Kenzaka, T., Naito, D. et al. “Pseudo aortoiliac bifurcation” leading to significant plaque shifting in the endovascular treatment of an aortoiliac bifurcation lesion: a case report. BMC Cardiovasc Disord 17, 179 (2017). https://doi.org/10.1186/s12872-017-0614-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-017-0614-2