Abstract
Background
Several studies found mid-regional pro-adrenomedullin (ProADM), the prohormone of the cardiovascular protein adrenomedullin, to be strongly associated with short-term mortality, mostly in the inpatient setting. We evaluated associations of ProADM levels with 10-year mortality in community-dwelling primary care patients with respiratory tract infections.
Methods
This is a post-hoc analysis using clinical and biomarker data of 134 primary care patients with respiratory tract infections. ProADM was measured on admission and after 7 days in batch-analysis. 10-year follow-up data was collected by GP, patient and relative tracing through phone interviews. We calculated Cox regression models and area under the receiver operating characteristics curves to assess associations of ProADM with 10-year all-cause mortality.
Results
During the 10-year follow-up 6% of included patients died. Median baseline ProADM blood levels (nmol/l) were significantly higher in non-survivors compared to survivors (0.5, IQR 0.4–1.3; vs. 0.2, IQR 0.1–0.5; p = 0.02) and showed a significant association with 10-year all-cause mortality in an age-adjusted cox regression model (HR: 2.5, 95%-CI: 1.0–6.1, p = 0.04). ProADM levels on day 7 showed similar results.
Conclusions
This posthoc analysis found an association of elevated ProADM blood levels and 10-year all-cause mortality in a primary care cohort with respiratory tract infections. Due to the methodological limitations including incomplete data regarding follow-up information and biomarker measurement, this study warrants validation in future larger studies.
Trial registration
Current Controlled Trials, SRCTN73182671
Similar content being viewed by others
Background
Prognostic blood biomarkers have generated clinical interest, resulting in therapeutic and diagnostic improvements [1]. A more personalized and targeted medicine offers opportunities to enhance treatment safety, efficacy as well as cost effectiveness. In this context, mid-regional pro-adrenomedullin (ProADM) provides prognostic information in various clinical settings and across different patient populations, predominantly suffering from cardiovascular and infectious diseases [2–8].
Adrenomedullin (ADM) is a 52-amino acid ringed peptide, belonging to the calcitonin peptide superfamily [9, 10]. It is produced ubiquitously by endothelial cells in cardiovascular, renal, pulmonary, cerebrovascular and endocrine tissues [11–13]. Studies reported several effects of ADM including vasodilatory, natriuretic, diuretic, anti-oxidative, anti-inflammatory, antimicrobial and metabolic effects [14–19]. Unfortunately, ADM measurement is challenging and not available outside the research setting. ProADM, the prohormone of ADM, however, is less biologically active, stable and commercially available [20].
Several studies found ProADM to be a strong and independent predictor of outcomes in patients with COPD [21], acute or chronic heart failure [22–26], community-acquired pneumonia [27, 28] and sepsis [29–32]. ProADM blood levels were also associated with morbidity after acute coronary syndrome [33–36], and predicted adverse outcomes in patients presenting to the emergency department (ED) with dyspnea [23, 37] or even nonspecific complaints [38]. Also, studies found association of ProADM with metabolic syndrome and its components, i.e. type 2 diabetes [39, 40].
Stratification of patients with lower respiratory infection based on a clinical algorithm including ProADM stratification tended to shorten length of hospital stay without an increase in adverse clinical outcome [41, 42].
In the setting of primary care, in smaller studies ProADM was associated with mortality in patients with urinary tract infections [43], type 2 diabetes [43–45] or heart failure [25, 46–49]. It is not known whether this marker helps to predict long-term risk, which could be useful for directing preventive measures. The aim of the current analysis was to investigate the ability of ProADM to predict long-term all-cause mortality and adverse outcome in a primary care cohort, visiting their general practitioner for respiratory infections.
Methods
Study design and setting
This is a post-hoc analysis investigating ProADM in a primary care cohort enrolled between December 2004 and April 2006 into the PARTI intervention trial (Procalcitonin-Guided Antibiotic Use vs. a Standard Approach for Acute Respiratory Tract Infections in Primary Care) [50, 51]. In brief, this randomized, multicenter, non-inferiority trial investigated the feasibility of a PCT-guided antibiotic therapy visiting their GP for a respiratory infection. The use of antibiotics was more or less strongly discouraged based on defined PCT-cutoff ranges. The standard approach followed evidence-based guidelines for use of antibiotics. The aim of the trial was to investigate safety and efficacy of using PCT levels to guide antibiotic therapy.
The initial study protocol, as well as the present follow-up trial, were approved by the local Ethics Committee of Basel (EKBB). Written informed consent was obtained from all participating physicians and patients.
Selection of participants
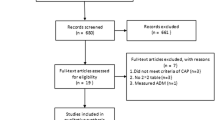
Initially, all patients with the diagnosis of upper or lower ARTI and the physician’s intention to prescribe antibiotics were included. Exclusion criteria were antibiotic use within the previous 28 days, psychiatric disorders or inability to give written informed consent, not being available for follow-up, not being fluent in German, severe immunosuppression, cystic fibrosis, active tuberculosis, and the need for immediate hospitalization. In this analysis, we included only patients, of which the 10-year follow-up and either baseline or day 7 ProADM levels were available.
Data collection and endpoints
The primary endpoint was defined as long-term 10-year all-cause mortality. Secondary outcomes were adverse outcomes including death, pulmonary embolism, and major adverse cardiac or cerebrovascular events (MACCE), which includes cardiac infarction, cardiac arrest, stroke and transient ischemic attack. In addition, we investigated new onset of diabetes within the follow-up period.
To verify outcomes, we performed follow-up telephone interviews with patients, relatives and/or general practitioners (GPs) 10 years after the baseline visit, using systematic questionnaires. Also, the register of death of the cantons Basel-Stadt and Basel-Land was consulted if no information about vital status was available.
Analysis of blood biomarkers
Blood samples were collected in the primary care centre from each patient on admission and after 7 days in ethylendiaminetetraacetic (EDTA) tubes and sent by courier to the central Laboratory of the University Hospital Basel for measurement of PCT. Leftover blood samples were frozen and stored at −80° for the later measurement of prognostic markers.
ProADM serum values were determined using a sandwich immunoassay with a functional interassay precision of 20% and an assay sensitivity assessed as being 0.12 nmol/L (B.R.A.H.M.S. Sevadil® LIA; B.R.A.H.M.S. GmbH, Hennigsdorf, Germany) [20].
Statistical analysis
We used STATA 12.1 (STATA Corp, College Station, TX; USA) and created univariable and bivariable (adjusting for age) as well as a multivariable model (adjusting for age, randomisation arm (PCT group), smoking history) cox regression analysis to calculate hazard ratios (HR) and area under the receiver operating characteristics curve (AUC) to investigate the predictive accuracy of Pro-ADM. We used a natural logarithm (base e) transformation of all biomarker data before entering into the statistical models to approximate normal distribution. Therefore, HR corresponds to a 2.72-fold increase in log transformed biomarker levels. To illustrate the predictive power we used Kaplan-Meier plots for proportion of survivors and proportion of events by quartiles of biomarker levels. Log-rank tests were performed to compare quartiles. P-values of <0.05 were considered to indicate statistical significance.
Results
Patient population
The initial cohort included 458 adult patients with an ARTI, of which 167 (36.5%) had ProADM blood samples available, while the other patients had not enough leftover sample for measurement of ProADM. A total of 134 (80.2%) of these patients could be contacted to assess long-term outcomes between April and August 2015 and were, thus, included in the final analysis. For 291 (63.5%) patients of the initial cohort no data concerning ProADM blood levels was available due to missing blood tube.
Table 1 shows baseline characteristics of the overall cohort as well as stratified by survival status. The median age was 42.0 years and 32.8% of patients were male. Median ProADM blood levels on admission were 0.3 nmol/l and 0.2 nmol/l on day 7. According to survival status, there were significant differences in age, comorbidities (chronic obstructive pulmonary disease), initial clinical classification of type of respiratory infection and nicotine consumption (pack-years). A comparison between the initial cohort and the patients available for 10-year follow-up is presented in Table 5 (see Appendix).
A similar 10-year post-hoc analysis of the prognostic biomarkers copeptin and MR-proANP, based on the same blood samples from the PARTI trial, has been published recently and showed that copeptin as well as MR-proANP were associated with 10-year all-cause mortality [52, 53].
Primary outcome: 10-year all-cause mortality
During the follow-up of 10 years (mean: 9.5 years), mortality was 6% (n = 8 patients). Median admission ProADM blood levels (nmol/l) were significantly higher in non-survivors compared to survivors (0.5, IQR 0.4–1.3 (n = 8); vs. 0.2, IQR 0.1–0.5 (n = 122); p = 0.02). Similar results were found at day 7 (0.4, IQR 0.2–0.7 (n = 7); vs. 0.2, IQR 0.1–0.4 (n = 116); p = 0.08).
Initial ProADM level showed a strong association with 10-year all-cause mortality in a univariable cox regression model (HR: 4.2, 95%-CI: 1.5, 11.5, p = 0.006). The result remained significant when adjusted for age (adjusted HR: 2.5, 95%-CI: 1.0–6.1, p = 0.043). There was no significant effect modification by randomisation arm (PCT group) or a positive history of smoking. Day seven ProADM levels showed in the univariable model a hazard ratio of borderline significance (HR: 2.7, 95%-CI: 1.0–7.4, p = 0.06).
The areas under the receiver operating curve (AUC) suggest fair accuracies of ProADM levels at baseline and on day seven (AUC: 0.74, 95%-CI: 0.54–0.94 and AUC: 0.70, 95%-CI: 0.48, 0.91).
We generated Kaplan-Meier curves to visualize the difference of survival between the highest quartile and lower three quartiles on admission and on day 7 (Figs. 1 and 2). The log-rank-test showed no significant increase in mortality when comparing the highest ProADM quartile to quartiles 1 to 3.
Secondary outcomes
During the 10-year follow-up, 9.7% of patients (n = 13) had an adverse outcome event including death, pulmonary embolism, and major adverse cardiac or cerebrovascular events (MACCE). At baseline, median ProADM levels (nmol/L) showed no statistically significant difference between patients with or without adverse outcome event (0.4, IQR 0.2–0.5; vs. 0.3, IQR 0.1–0.5; p = 0.15).
Cox regression models found borderline significant associations between initial and ProADM levels and adverse outcome, with an AUC of 0.62 (Table 2). When adjusted for age (HR: 1.4, 95%-CI: 0.8 to 2.5), p = 0.273), there was no significant association found. As well, there was no significant effect modification by randomisation arm (PCT group) or a positive history of smoking.
Significance tests for follow-up ProADM levels showed similar results.
Kaplan-Meier curves (Figs. 3 and 4), with patients stratified based on ProADM quartiles, illustrate the slightly higher event rates in patients in the highest ProADM quartile.
Further, no association between ProADM and MACCE or new-onset diabetes mellitus was found (see Appendix).
Discussion
Within this prospective, observational 10-year follow-up study of a small cohort of community-dwelling ARTI patients, we found ProADM on admission and after 7 days to be an age-independent predictor for all-cause mortality. No significant associations of ProADM and secondary outcomes were found, namely adverse outcome, MACCE and new onset of diabetes.
Our results are in-line with previous research reporting associations of ProADM and short-term mortality in inpatients [2,3,4, 21–38] as well as in primary care populations [25, 43–49]. Importantly, also in long-term follow-up over 10 years the prognostic accuracy remained stable over time. Thus, based on this study and previous research, ProADM is a short- and long-term valid prognostic marker for patients from the community who may benefit from preventive measures. Our study suggests that ProADM should be evaluated in future long-term studies in outpatients assessing the accuracy of clinical scores (e.g., Pneumonia Severity Index, CURB65 (confusion, uremia, respiratory rate, blood pressure, age at least 65 years) score or Framingham score) in combination with novel markers to predict long-term outcome in this setting and direct individual use of medication or even hospital admission.
Although there is no clear understanding why an increase in ProADM points to increased mortality risk, existing data suggests that elevated ProADM levels reflect disease severity and endothelial and cardiovascular dysfunction [11–19]. Also, higher ADM levels increases cardiac output, induces hypotension and vasodilation, and increases glomerular filtration rate and fractional sodium excretion [10, 19, 54], thereby inducing a reduction in cardiac pre- and afterload [20]. Thus, the involvement of ADM in several pathological disease states and comorbidities might explain the associations found in this and previous studies.
Median ProADM blood levels in our outpatient cohort were 0.3 nmol/l on admission and 0.2 nmol/l on day 7 and thus significantly lower compared to other hospitalized patient cohorts. The AtheroGene study found median ProADM levels of around 0.5 and 0.6 nmol/L in patients with stable angina and acute coronary syndrome [35]. The LAMP study reported median concentration of 0.73 nmol/L in patients with myocardial infarction [36], while the GISSI study found a median ProADM concentration of 0.75 nmol/L in patients with chronic heart failure [26]. Analysis of a presumably healthy subset in a large outpatient cohort (n = 5258) lead to a reference interval of 0.23—0.64 nmol/L [18, 55]. These differences demonstrate that levels of ProADM need to be adapted to the specific clinical setting to be interpreted in a meaningful way.
Interestingly, although several previous studies have suggested that ProADM was also associated with other adverse outcomes in addition to all-cause mortality [26, 27, 33–36, 44–46], we did not find such statistically significant association with the incidence of our secondary combined endpoint including pulmonary embolism and MACCE. Also, in contrast to another study [39], our analysis did not find an association of ProADM with new onset diabetes mellitus. This may be due to the small number of events in our generally healthy population with a low burden of comorbidities and thus low power of our analysis. Further, the respiratory infection of patients during the initial trial may have had an influence on ProADM levels. Thus, similar to lipid levels, [56] this marker may be best analyzed during stable conditions for the purpose of long-term risk assessment.
The main strengths of this study include the 10 years of follow-up, the participation of multiple GP practices, and the community sample of patients with ARTI of different severity representative for patients mainly treated in primary care. Nonetheless, we are aware of several limitations. First, this is a secondary analysis of a previous trial and baseline risk assessment is incomplete as is the availability of ProADM levels in the cohort. For 291 (63.5%) patients no data concerning ProADM blood levels was available, because blood sampling was only done in a sub fraction of the overall cohort during a certain time period. Selection bias is thus possible. Second, due to the long follow-up period, a recall bias has to be considered. Further, no information was available on the cause of death, when patients were tracked through the register of deaths. Third, our sample was small and we observed only few events for the analysis of the relationship between ProADM levels and adverse outcomes.
Conclusion
This posthoc analysis found an association of elevated ProADM blood levels and 10-year all-cause mortality in a primary care cohort with respiratory tract infections. Due to the methodological limitations including incomplete data regarding follow-up information and biomarker measurement, this study warrants validation in future larger studies. If validated, ProADM may help to risk stratify patients and thereby allows to improve allocation of health care resources and preventive measures.
Abbreviations
- ARTI:
-
acute respiratory tract infection
- AUC:
-
area under the receiver operating characteristic curve
- CI:
-
confidence interval
- COPD:
-
chronic obstructive pulmonary disease
- CV:
-
cardiovascular
- DM:
-
diabetes mellitus
- ED:
-
emergency department
- EKBB:
-
Ethics Committee of Basel (Switzerland)
- GP:
-
general practitioner
- HR:
-
hazard ratio
- IQR:
-
interquartile range (25th–75th percentiles)
- MACCE:
-
major adverse cardiac and cerebrovascular event
- NPV:
-
negative predictive value
- PCT:
-
procalcitonin
- PPV:
-
positive predictive value
- ProADM:
-
MR-pro-Adrenomedullin
- SD:
-
standard deviation
References
Schuetz P, Aujesky D, Muller C, Muller B. Biomarker-guided personalised emergency medicine for all - hope for another hype? Swiss Med Wkly. 2015;145:w14079.
Schuetz P, Litke A, Albrich WC, Mueller B. Blood biomarkers for personalized treatment and patient management decisions in community-acquired pneumonia. Curr Opin Infect Dis. 2013;26(2):159–67.
Albrich WC, Dusemund F, Ruegger K, Christ-Crain M, Zimmerli W, Bregenzer T, et al. Enhancement of CURB65 score with proadrenomedullin (CURB65-a) for outcome prediction in lower respiratory tract infections: derivation of a clinical algorithm. BMC Infect Dis. 2011;11:112.
Schuetz P, Wolbers M, Christ-Crain M, Thomann R, Falconnier C, Widmer I, et al. Prohormones for prediction of adverse medical outcome in community-acquired pneumonia and lower respiratory tract infections. Crit Care. 2010;14(3):R106.
Melander O, Newton-Cheh C, Almgren P, Hedblad B, Berglund G, Engstrom G, et al. Novel and conventional biomarkers for prediction of incident cardiovascular events in the community. JAMA. 2009;302(1):49–57.
Gombos T, Forhecz Z, Pozsonyi Z, Wallentin S, Papassotiriou J, Kunde J, et al. Adrenomedullin and endothelin-1 are related to inflammation in chronic heart failure. Inflamm Res. 2009;58(6):298–305.
Schuetz P, Christ-Crain M, Morgenthaler NG, Struck J, Bergmann A, Muller B. Circulating precursor levels of endothelin-1 and adrenomedullin, two endothelium-derived, counteracting substances, in sepsis. Endothelium. 2007;14(6):345–51.
Schuetz P, Hausfater P, Amin D, Amin A, Haubitz S, Faessler L, et al. Biomarkers from distinct biological pathways improve early risk stratification in medical emergency patients: the multinational, prospective, observational TRIAGE study. Crit Care. 2015;19(1):377.
Kitamura K, Sakata J, Kangawa K, Kojima M, Matsuo H, Eto T. Cloning and characterization of cDNA encoding a precursor for human adrenomedullin. Biochem Biophys Res Commun. 1993;194(2):720–5.
Kitamura K, Kangawa K, Eto T. Adrenomedullin and PAMP: discovery, structures, and cardiovascular functions. Microsc Res Tech. 2002;57(1):3–13.
Jougasaki M, Burnett JC Jr. Adrenomedullin: potential in physiology and pathophysiology. Life Sci. 2000;66(10):855–72.
Sugo S, Minamino N, Shoji H, Kangawa K, Kitamura K, Eto T, et al. Production and secretion of adrenomedullin from vascular smooth muscle cells: augmented production by tumor necrosis factor-alpha. Biochem Biophys Res Commun. 1994;203(1):719–26.
Isumi Y, Shoji H, Sugo S, Tochimoto T, Yoshioka M, Kangawa K, et al. Regulation of adrenomedullin production in rat endothelial cells. Endocrinology. 1998;139(3):838–46.
Kitamura K, Kangawa K, Kojima M, Ichiki Y, Matsuo H, Eto T. Complete amino acid sequence of porcine adrenomedullin and cloning of cDNA encoding its precursor. FEBS Lett. 1994;338(3):306–10.
Temmesfeld-Wollbruck B, Hocke AC, Suttorp N, Hippenstiel S. Adrenomedullin and endothelial barrier function. Thromb Haemost. 2007;98(5):944–51.
Elsasser TH, Kahl S. Adrenomedullin has multiple roles in disease stress: development and remission of the inflammatory response. Microsc Res Tech. 2002;57(2):120–9.
Allaker RP, Grosvenor PW, McAnerney DC, Sheehan BE, Srikanta BH, Pell K, et al. Mechanisms of adrenomedullin antimicrobial action. Peptides. 2006;27(4):661–6.
Smith JG, Newton-Cheh C, Hedblad B, Struck J, Morgenthaler NG, Bergmann A, et al. Distribution and correlates of midregional proadrenomedullin in the general population. Clin Chem. 2009;55(8):1593–5.
Samson WK. Adrenomedullin and the control of fluid and electrolyte homeostasis. Annu Rev Physiol. 1999;61:363–89.
Morgenthaler NG, Struck J, Alonso C, Bergmann A. Measurement of midregional proadrenomedullin in plasma with an immunoluminometric assay. Clin Chem. 2005;51(10):1823–9.
Schuetz P, Marlowe RJ, Mueller B. The prognostic blood biomarker proadrenomedullin for outcome prediction in patients with chronic obstructive pulmonary disease (COPD): a qualitative clinical review. Clin Chem Lab Med. 2015;53(4):521–39.
Maisel A, Mueller C, Nowak R, Peacock WF, Landsberg JW, Ponikowski P, et al. Mid-region pro-hormone markers for diagnosis and prognosis in acute dyspnea: results from the BACH (biomarkers in acute heart failure) trial. J Am Coll Cardiol. 2010;55(19):2062–76.
Shah RV, Truong QA, Gaggin HK, Pfannkuche J, Hartmann O, Januzzi JL Jr. Mid-regional pro-atrial natriuretic peptide and pro-adrenomedullin testing for the diagnostic and prognostic evaluation of patients with acute dyspnoea. Eur Heart J. 2012;33(17):2197–205.
Gegenhuber A, Struck J, Dieplinger B, Poelz W, Pacher R, Morgenthaler NG, et al. Comparative evaluation of B-type natriuretic peptide, mid-regional pro-A-type natriuretic peptide, mid-regional pro-adrenomedullin, and Copeptin to predict 1-year mortality in patients with acute destabilized heart failure. J Card Fail. 2007;13(1):42–9.
von Haehling S, Filippatos GS, Papassotiriou J, Cicoira M, Jankowska EA, Doehner W, et al. Mid-regional pro-adrenomedullin as a novel predictor of mortality in patients with chronic heart failure. Eur J Heart Fail. 2010;12(5):484–91.
Masson S, Latini R, Carbonieri E, Moretti L, Rossi MG, Ciricugno S, et al. The predictive value of stable precursor fragments of vasoactive peptides in patients with chronic heart failure: data from the GISSI-heart failure (GISSI-HF) trial. Eur J Heart Fail. 2010;12(4):338–47.
Christ-Crain M, Morgenthaler NG, Stolz D, Muller C, Bingisser R, Harbarth S, et al. Pro-adrenomedullin to predict severity and outcome in community-acquired pneumonia [ISRCTN04176397]. Crit Care. 2006;10(3):R96.
Huang DT, Angus DC, Kellum JA, Pugh NA, Weissfeld LA, Struck J, et al. Midregional proadrenomedullin as a prognostic tool in community-acquired pneumonia. Chest. 2009;136(3):823–31.
Suberviola B, Castellanos-Ortega A, Llorca J, Ortiz F, Iglesias D, Prieto B. Prognostic value of proadrenomedullin in severe sepsis and septic shock patients with community-acquired pneumonia. Swiss Med Wkly. 2012;142:w13542.
Al Shuaibi M, Bahu RR, Chaftari AM, Al Wohoush I, Shomali W, Jiang Y, et al. Pro-adrenomedullin as a novel biomarker for predicting infections and response to antimicrobials in febrile patients with hematologic malignancies. Clin Infect Dis. 2013;56(7):943–50.
Courtais C, Kuster N, Dupuy AM, Folschveiller M, Jreige R, Bargnoux AS, et al. Proadrenomedullin, a useful tool for risk stratification in high pneumonia severity index score community acquired pneumonia. Am J Emerg Med. 2013;31(1):215–21.
Christ-Crain M, Morgenthaler NG, Struck J, Harbarth S, Bergmann A, Muller B. Mid-regional pro-adrenomedullin as a prognostic marker in sepsis: an observational study. Crit Care. 2005;9(6):R816–24.
Dhillon OS, Khan SQ, Narayan HK, Ng KH, Struck J, Quinn PA, et al. Prognostic value of mid-regional pro-adrenomedullin levels taken on admission and discharge in non-ST-elevation myocardial infarction: the LAMP (Leicester acute myocardial infarction peptide) II study. J Am Coll Cardiol. 2010;56(2):125–33.
Behnes M, Papassotiriou J, Walter T, Fiedler E, Sauer T, Lang S, et al. Long-term prognostic value of mid-regional pro-adrenomedullin and C-terminal pro-endothelin-1 in patients with acute myocardial infarction. Clin Chem Lab Med. 2008;46(2):204–11.
Wild PS, Schnabel RB, Lubos E, Zeller T, Sinning CR, Keller T, et al. Midregional proadrenomedullin for prediction of cardiovascular events in coronary artery disease: results from the AtheroGene study. Clin Chem. 2012;58(1):226–36.
Khan SQ, O'Brien RJ, Struck J, Quinn P, Morgenthaler N, Squire I, et al. Prognostic value of midregional pro-adrenomedullin in patients with acute myocardial infarction: the LAMP (Leicester acute myocardial infarction peptide) study. J Am Coll Cardiol. 2007;49(14):1525–32.
Maisel A, Mueller C, Nowak RM, Peacock WF, Ponikowski P, Mockel M, et al. Midregion prohormone adrenomedullin and prognosis in patients presenting with acute dyspnea: results from the BACH (biomarkers in acute heart failure) trial. J Am Coll Cardiol. 2011;58(10):1057–67.
Nickel CH, Messmer AS, Geigy N, Misch F, Mueller B, Dusemund F, et al. Stress markers predict mortality in patients with nonspecific complaints presenting to the emergency department and may be a useful risk stratification tool to support disposition planning. Acad Emerg Med Off J Soc Acad Emerg Med. 2013;20(7):670–9.
Seissler J, Feghelm N, Then C, Meisinger C, Herder C, Koenig W, et al. Vasoregulatory peptides pro-endothelin-1 and pro-adrenomedullin are associated with metabolic syndrome in the population-based KORA F4 study. Eur J Endocrinol. 2012;167(6):847–53.
Del Ry S, Cabiati M, Bianchi V, Caponi L, Di Cecco P, Marchi B, et al. Mid-regional-pro-adrenomedullin plasma levels are increased in obese adolescents. Eur J Nutr. 2015;
Schuetz P, Hausfater P, Amin D, Amin A, Haubitz S, Faessler L, et al. Biomarkers from distinct biological pathways improve early risk stratification in medical emergency patients: the multinational, prospective, observational TRIAGE study. Crit Care. 2015;19:377.
Nickel CH, Messmer AS, Ghanim L, Ilsemann-Karakoumis J, Giersdorf S, Hertel S, et al. Adrenomedullin for risk stratification of emergency patients with nonspecific complaints: an interventional multicenter pilot study. Medicine. 2016;95(1):e2395.
van der Starre WE, Zunder SM, Vollaard AM, van Nieuwkoop C, Stalenhoef JE, Delfos NM, et al. Prognostic value of pro-adrenomedullin, procalcitonin and C-reactive protein in predicting outcome of febrile urinary tract infection. Clin Microbiol Infect. 2014;20(10):1048–54.
Landman GW, van Dijk PR, Drion I, van Hateren KJ, Struck J, Groenier KH, et al. Midregional fragment of proadrenomedullin, new-onset albuminuria, and cardiovascular and all-cause mortality in patients with type 2 diabetes (ZODIAC-30). Diabetes Care. 2014;37(3):839–45.
Maier C, Clodi M, Neuhold S, Resl M, Elhenicky M, Prager R, et al. Endothelial markers may link kidney function to cardiovascular events in type 2 diabetes. Diabetes Care. 2009;32(10):1890–5.
Alehagen U, Dahlstrom U, Rehfeld JF, Goetze JP. Pro-A-type natriuretic peptide, proadrenomedullin, and N-terminal pro-B-type natriuretic peptide used in a multimarker strategy in primary health care in risk assessment of patients with symptoms of heart failure. J Card Fail. 2013;19(1):31–9.
Holmager P, Schou M, Egstrup M, Gustafsson I, Goetze JP, Gustafsson F, et al. The influence of diabetes mellitus on midregional proadrenomedullin concentrations and prognostic value in heart failure outpatients. J Card Fail. 2015;21(3):250–7.
Xue Y, Taub P, Iqbal N, Fard A, Clopton P, Maisel A. Mid-region pro-adrenomedullin adds predictive value to clinical predictors and Framingham risk score for long-term mortality in stable outpatients with heart failure. Eur J Heart Fail. 2013;15(12):1343–9.
Adlbrecht C, Hulsmann M, Strunk G, Berger R, Mortl D, Struck J, et al. Prognostic value of plasma midregional pro-adrenomedullin and C-terminal-pro-endothelin-1 in chronic heart failure outpatients. Eur J Heart Fail. 2009;11(4):361–6.
Briel M, Schuetz P, Mueller B, Young J, Schild U, Nusbaumer C, et al. Procalcitonin-guided antibiotic use vs a standard approach for acute respiratory tract infections in primary care. Arch Intern Med. 2008;168(18):2000–7. discussion 7-8
Briel M, Christ-Crain M, Young J, Schuetz P, Huber P, Periat P, et al. Procalcitonin-guided antibiotic use versus a standard approach for acute respiratory tract infections in primary care: study protocol for a randomised controlled trial and baseline characteristics of participating general practitioners [ISRCTN73182671]. BMC Fam Pract. 2005;6:34.
Odermatt J, Bolliger R, Hersberger L, Ottiger M, Christ-Crain M, Briel M, et al. Copeptin predicts 10-year all-cause mortality in community patients: a 10-year prospective cohort study. Clin Chem Lab Med. 2016;54(10):1681–90.
Odermatt J, Hersberger L, Bolliger R, Graedel L, Christ-Crain M, Briel M, et al. The natriuretic peptide MR-proANP predicts all-cause mortality and adverse outcome in community patients: a 10-year follow-up study. Clin Chem Lab Med. 2017. doi:10.1515/cclm-2016-0760.
Parkes DG, May CN. Direct cardiac and vascular actions of adrenomedullin in conscious sheep. Br J Pharmacol. 1997;120(6):1179–85.
Neumann JT, Tzikas S, Funke-Kaiser A, Wilde S, Appelbaum S, Keller T, et al. Association of MR-proadrenomedullin with cardiovascular risk factors and subclinical cardiovascular disease. Atherosclerosis. 2013;228(2):451–9.
Gruber M, Christ-Crain M, Stolz D, Keller U, Muller C, Bingisser R, et al. Prognostic impact of plasma lipids in patients with lower respiratory tract infections - an observational study. Swiss Med Wkly. 2009;139(11–12):166–72.
Acknowledgements
We are grateful to the physicians, their staff and patients who participated in the PARTI trial and the follow-up data collection. We would also like to thank the staff of the central laboratory of the University Hospital Basel for their assistance and technical support.
Funding
The initial investigator-initiated PARTI trial was sponsored by a grant from the Swiss National Science Foundation (3300C0–107,772) and by the Association for the Promotion of Science and Postgraduate Training of the University Hospital Basel. Brahms AG provided assay and kit material related to the study.
Availability of data and materials
The data set supporting the results of this article is included within the article and its additional files.
Author information
Authors and Affiliations
Contributions
Mr. JO and Mr. MM contributed equally to this study. Mr. JO, Mr. MM and Dr. PS had full access to all of the data in the study and take responsibility for the integrity of the data. Mr. JO, Mr. MM and Dr. PS performed the statistical work and drafted the manuscript. All authors helped to interpret the findings, read and revised the manuscript critically for important intellectual content, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The initial study protocol, as well as the present follow-up trial, were approved by the local Ethics Committee of Basel (EKBB). Written informed consent was obtained from all participating physicians and patients.
Consent for publication
Not applicable.
Competing interests
This investigator-initiated PARTI trial was sponsored by a grant from the Swiss National Science Foundation (SNSF Professorship, PP00P3_150531), by the Association for the Promotion of Science and Postgraduate Training of the University Hospital Basel and the Forschungsrat of the Kantonsspital Aarau (1410.000.058 and 1410.000.044). Brahms AG provided assay and kit material related to the study.
Dr. Schuetz, and Dr. Christ-Crain were supported by funds of the Freiwillige Akademische Gesellschaft, the Department of Endocrinology, Diabetology and Clinical Nutrition, and the Department of Clinical Chemistry, all Basel, Switzerland.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Drs. Christ-Crain, Kutz, Mueller and Schuetz received support from BRAHMS to attend meetings and fulfilled speaking engagements. Drs. Schuetz, Christ-Crain and Mueller received support from bioMérieux to attend meetings and fulfilled speaking engagements. Dr. Mueller has served as a consultant and received research support from BRAHMS and bioMérieux. All other authors declare that the answer to the questions on the competing interest form are all “No” and therefore have nothing to declare.
All authors confirm that they do not have a conflict of interest associated with this manuscript.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Odermatt, J., Meili, M., Hersberger, L. et al. Pro-Adrenomedullin predicts 10-year all-cause mortality in community-dwelling patients: a prospective cohort study. BMC Cardiovasc Disord 17, 178 (2017). https://doi.org/10.1186/s12872-017-0605-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12872-017-0605-3