Abstract
Background
SNF-related Kinase 1 (SnRK1) is a key component of the cell signaling network. SnRK1 is known to respond to a wide variety of stresses, but its exact role in salt stress response and tolerance is still largely unknown.
Results
In this study, we reported that overexpression of the gene encoding the α subunit of Prunus persica SnRK1 (PpSnRK1α) in tomato could improve salt stress tolerance. The increase in salt stress tolerance in PpSnRK1α-overexpressing plants was found to correlate with increased PpSnRK1α expression level and SnRK1 kinase activity. And PpSnRK1α overexpression lines exhibited a lower level of leaf damage as well as increased proline content and reduced malondialdehyde (MDA) compared with wild-type (WT) lines under salt stress. Furthermore, PpSnRK1α enhanced reactive oxygen species (ROS) metabolism by increasing the expression level of antioxidase genes and antioxidant enzyme activities. We further sequenced the transcriptomes of the WT and three PpSnRK1α overexpression lines using RNA-seq and identified about 1000 PpSnRK1α-regulated genes, including many antioxidant enzymes, and these genes were clearly enriched in the MAPK signaling pathway (plant), plant-pathogen interactions and plant hormone signaling transduction and can respond to stimuli, metabolic processes, and biological regulation. Furthermore, we identified the transcriptional levels of several salt stress-responsive genes, SlPP2C37, SlPYL4, SlPYL8, SlNAC022, SlNAC042, and SlSnRK2 family were altered significantly by PpSnRK1α, signifying that SnRK1α may be involved in the ABA signaling pathway to improve tomato salt tolerance. Overall, these findings provided new evidence for the underlying mechanism of SnRK1α conferment in plant salt tolerance phenotypes.
Conclusions
Our findings demonstrated that plant salt stress resistance can be affected by the regulation of the SnRK1α. Further molecular and genetic approaches will accelerate our knowledge of PpSnRK1α functions, and inform the genetic improvement of salt tolerance in tomato through genetic engineering and other related strategies.
Similar content being viewed by others
Background
Currently, salt stress affects over 6% of the global area (more than 800 million hectares of land), and the infected area continues to increase [1]. In plants, high salt can affect the absorption of water and nutrients and reduce photosynthesis, thereby inhibiting plant growth and causing yield loss. And at the cellular level, high salt can disturb normal metabolism and cause “physiological drought”, ionic toxicity or causing complex secondary damages to proteins, nucleic acids, and other macromolecular substances [2].
Fundamentally, the salt tolerance of plants is conferred by tolerance genes. The isolation of novel tolerance genes and effective identification of salt-resistant germplasm are key strategies for improving salt tolerance in plants [3]. Recently, extensive genetic and molecular studies using loss-of-function mutants and overexpression lines have been carried out to investigate the molecular basis of salt tolerance in model plants and crop species. It has been reported that protein kinases of the mitogen activated protein kinase (MAPK), Calcineurin B-like-interacting protein kinase (CIPK) and SNF1-related protein kinases family(SnRKs) families and many transcription factors (TFs), such as NAC(NAM, ATAF1,2, and CUC2), AP2/ERF, MYB, WRKY, bZIP, were found to play important roles in environmental stress responses [4,5,6,7,8,9,10,11,12]. Therefore, screening for salt-tolerant genes via molecular studies is a necessity for protecting the plants against salt stress.
The structure and function of SnRK1 are similar to those of the yeast SNF1 and mammalian AMPK [13]. SnRK1 holoenzyme is a heterotrimer comprising a catalytic α subunit, a regulatory γ or βγ subunit, and β subunit as a scaffold connecting α and γ subunits [13]. Phosphorylation of the conserved threonine on the T-loop of the catalytic α subunit is required for SNF1/AMPK/SnRK1 to remain active [14,15,16,17].
SnRK1 is involved in sucrose and lipid metabolism in plants and can respond to stress signals under stress conditions, thereby regulating plant growth and development [18]. For example, increased SNF1 catalytic activity and phosphorylation of the conserved Thr-210 in the activation loop occur in yeast cells exposed to sodium or oxidative stress, indicating that similar to AMPK, SNF1 is activated in response to these stresses [19]. Moreover, it has been reported that SnRK1 activity is required for flooding resistance in Arabidopsis [20]. Likewise, SnRK1 is an essential kinase induced by autophagy under various stress conditions in Arabidopsis [21].
The sucrose nonfermenting-1-related protein kinases(SnRKs)family can respond to stress through regulating multiple signaling pathways via cascade amplification of the stress signal and initiate a stress response. The SnRKs family can be further subdivided into three subfamilies—SnRK1, SnRK2, and SnRK3—according to the differences in tertiary structures [22]. In Arabidopsis, SnRK2.2/SnRK2.4/SnRK2.6 are important regulators of ABA signaling in response to abiotic stress [23], and SnRK3 indirectly participates in ABA signaling by interacting with the regulatory factors in the ABA signaling pathway, thereby enhancing stress tolerance in plants. For example, ABI2 interacts strongly with SnRK3.11/SnRK3.13/SnRK3.15 to regulate the expression of salt stress-related genes, rendering the plants more sensitive to the stress signal and enhancing salt tolerance [24, 25]. In Arabidopsis, SnRK1 is negatively regulated by the ABA-insensitive 1 (ABI1) and protein phosphatase 2C A group (PP2CA) [26]. Therefore, we speculate that SnRK1 is involved in abiotic stress response via ABA signaling or other pathways.
Peach (Prunus persica) is a unique plant species, probably originated from China. It contains abundant valuable genetic resources that can be used to study and improve stress tolerance in plants [27]. In this study, the full-length Prunus persica SnRK1α (PpSnRK1α) sequence was obtained from Prunus persica (Linn.) Batsch. The wild-type (WT) and three transgenic tomato seedlings overexpressing PpSnRK1α (OE-1, OE-4, and OE-7) in WT were used. In order to study the possible regulatory mechanism of SnRK1 under salt stress, we compared the phenotype of the WT and transgenic tomato plants under salt stress and analyzed the physiological indexes, including the metabolic capacity of ROS, and ability to resist osmotic stress. Moreover, we sequenced the transcriptomes of the WT and three PpSnRK1α overexpression lines using RNA-seq and identified approximately 1000 differentially expressed genes (DEGs). We further examined the expression levels of salt stress-related genes that significantly correlated with SnRK1 and validated the interactions between SnRK1 and known ABA receptors, which provide evidence for a role of SnRK1 in salt stress response, signifying that SnRK1α may be involved in the ABA signaling pathway or reactive oxygen (ROS) metabolism to improve tomato salt tolerance. Overall, our results demonstrate that the overexpression of PpSnRK1α is beneficial for enhancing salt tolerance in plants.
Results
PpSnRK1α overexpression lines have higher SnRK1 activity and increased salt tolerance
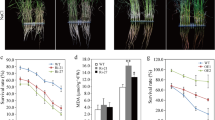
In order to further identify the difference between WT and PpSnRK1α-overexpressing tomatoes lines (PpSnRK1αoe), firstly we measured the expression levels of PpSnRK1α in overexpression lines (OE-1, OE-4, and OE-7) and WT. The results are shown in Fig. 1a. PpSnRK1αoe showed significant higher SnRK1α expression levels than WT, and SnRK1α expression levels were relatively higher in OE-1 and OE-4 than that of OE-7 (Fig. 1a, Additional file 1). Secondly, the SnRK1 activity of three PpSnRK1αoe significantly increased following two hours of the salt treatment and was 11.9–17.8% higher than that of the WT (Fig. 1b). However, SnRK1 activity declined after 24 h of high-salinity treatment both WT and PpSnRK1αoe. Thirdly, the relative electronic conductance of OE-1, OE-4, OE-7, and WT were 64.84, 60.43, 64.17, and 88.49%, respectively, after 12 days of salt treatment (Fig. 1c). In general, PpSnRK1α overexpression lines exhibited a lower level of cell damage than the WT. These observations were further validated by the Evans blue staining experiment (Fig. 1d). Thus, overexpression of PpSnRK1α increased the salt tolerance of plants, and the level of improvement was proportional to the level of PpSnRK1α gene expression and SnRK1 activity. Together, these results evidenced the role of PpSnRK1α overexpression in enhancing salt tolerance in tomato.
PpSnRK1α overexpression lines had higher SnRK1 activity and increased salt tolerance. a Relative expression level of SnRK1 (PCR was performed using homologous and specific fragments of PpSnRK1 (Prunus persica) and SlSnRK1 (Solanum lycopersicum) between WT plants and PpSnRK1α overexpressing plants). This picture was cropped for simplicity and intuitiveness, the original pictures were in Additional file 1b Determination of SnRK1 activity c REC of the WT and PpSnRK1α overexpression lines d Evans blue stain. Note: in b and c, the data are represented as means ± standard error (S.E.) of three biological replicates
PpSnRK1α regulates membrane lipid peroxidation and proline accumulation in response to salt stress
To further determine if overexpression of PpSnRK1α attenuate the degree of peroxidation of the cell membrane and accumulate proline [28] to resist salt stress, we monitored changes in the MDA content and proline content in both WT and PpSnRK1αoe before and after the salt treatment. No significant difference in MDA content was observed between the PpSnRK1αoe and WT plants under normal condition, Whereas, the MDA content in PpSnRK1αoe was obviously lower than that in the WT after 12 days of high salinity treatment (Fig. 2a). On the other hand, PpSnRK1αoe exhibited a more significant increase of proline content than the WT plants after 12 days of control treatment (Fig. 2b). Furthermore, proline contents accumulated to higher levels in PpSnRK1αoe than in WT under salt stress (Fig. 2b). However, the total soluble sugar content also was determined and found that there was a limited difference in soluble sugar levels between PpSnRK1αoe and WT (Fig. 2c). Overall, these results indicate that PpSnRK1α overexpression leads to a decrease in the degree of cell membrane peroxidation and the accumulation of proline in response to salt stress.
MDA, soluble sugar and proline contents in the WT and PpSnRK1α overexpression lines with and without the salt treatment. a MDA content b proline content c soluble sugar content. Three biological replicates were analyzed for each sample, and the data are represented as means ± S.E. of three technical repeats
PpSnRK1α overexpression lines exhibit reduced ROS content and higher antioxidant enzyme activities under salt stress
To further investigate the mechanism of how PpSnRK1α overexpression lines enhances salt tolerance in tomato, the correlation between overexpression of PpSnRK1α and reactive oxygen metabolism in plants and the differences in antioxidant enzyme activities as well as ROS content between the WT and transgenic plants were studied. After 12 days of the salt treatment, the overexpression of PpSnRK1α resulted in a significant decrease (17–26%) in the O2− content, compared with the level in WT plants (Fig. 2a). Consistent with these observations, NBT staining revealed that the transgenic leaves were less damaged than those of the WT under salt stress (Fig. 2b).
Under the normal growth condition, Superoxide dismutase (SOD), Catalase (CAT), Peroxisome (POD) activities of the transgenic lines were higher than those of the WT (Fig. 2c, e, f). Furthermore, the increases of SOD and POD activities in PpSnRK1α overexpression lines were much more evident, 62.9–96.7% and 8.0–15.8% increases than WT after 12 days of high salinity treatment (Fig. 2e, f). What’s more, PpSnRK1αoe exhibited higher CAT activity than the WT plants under salt stress. This result was consistent with those of the DAB staining assay, which indicated that transgenic plants have lower hydrogen peroxide content and higher CAT activity than WT (Fig. 2c, d). These results indicated that PpSnRK1α may play a role in antioxidant systems to protect plants under salt stress.
Overexpression of PpSnRK1α alter the expression level of SnRK2 family genes (SnRK2.1-SnRK2.7) and antioxidase genes (SOD, POD, and CAT1) in tomato
The expression levels of SOD, POD, and CAT1 by Quantitative Real-Time PCR (qRT-PCR) were detected both in PpSnRK1αoe and WT to further understand whether SnRK1 alters the expression of superoxide gene at the genetic level. According to Fig. 4, the transcript levels of genes encoding antioxidant enzymes (SOD, POD, and CAT) were the highest (6.839, 4.705 and 4.421 times than WT, respectively) among others in PpSnRK1α overexpression plants under high salinity condition (Fig. 4). Therefore, it can be seen that the overexpression of PpSnRK1α increased the expression level of the antioxidant enzyme genes at the genetic or transcriptional level.
There are seven Solanum lycopersicum SnRK2 genes (SlSnRK2s) that are homologous to the Arabidopsis SnRK2.2/SnRK2.3/SnRK2.6 genes in tomato [29]. In PpSnRK1α overexpression plants, the expression level of SnRK2.1 is not significantly changed, and the expression of SlSnRK2.2, SlSnRK2.3 and SlSnRK2.5 were inhibited regardless of whether the salt stress was present or not (Fig. 4). By contrast, the expression level of SlSnRK2.4 was increased in PpSnRK1αoe under both conditions (Fig. 4). However, the expression level of SlSnRK2.7 was inhibited under normal conditions but increased under salt stress (Fig. 4). Therefore, the overexpression of PpSnRK1α complexly affects the expression level of SlSnRK2.
Transcriptomic analysis of WT and PpSnRK1α overexpression plants
To further evaluate the molecular mechanism of how PpSnRK1α overexpression confers salt tolerance on a broader scale, the differences in gene expression profile between three PpSnRK1αoe (OE-1, OE-4, and OE-7) and WT plants under normal condition were analyzed. Three biological replicates of the WT and three transgenic lines were sequenced using the Illumina platform, and approximately 40 to 51 million high-quality reads (with Fast QC quality score > 36) were obtained for each biological replicate (Additional file 2). Of all reads obtained, 92 to 94% could be mapped to a unique chromosomal position. The expression level of each transcript in the sample was represented by Fragments Per Kilobase of transcript per Million fragments mapped (FPKM). A high linear correlation was observed among the three replicates of the same sample, suggesting small differences among replicates (Additional file 3). Differentially expressed genes (DEGs) were identified by comparing the transcriptomes of the WT and transgenic plants [30, 31]. We identified a total of 1009 DEGs, among which 668 were upregulated and 341 were downregulated, in PpSnRK1α overexpression plants compared with the WT (Fig. 5a). These results revealed that the expression of a large number of genes was altered upon PpSnRK1α overexpression, and these genes are either directly or indirectly regulated by SnRK1.
To test whether SnRK1α overexpression relieves severe effects of salt by modulating the expression of salt-responsive genes, functional annotations of both up-regulated and down-regulated genes and KEGG pathway enrichment analysis in PpSnRK1αoe were performed (Additional file 4; Fig. 5b; Additional file 5). MAPK (mitogen activated protein kinase) signaling pathway–plant, plant-pathogen interaction, and plant hormone signal transduction were main KEGG pathways that were regulated in PpSnRK1αoe. Then GO terms were assigned to the DEGs. A number of genes were involved in various cellular component, molecular function, and biological processes (Fig. 5c), of which 1714 GO terms were enriched among the upregulated DEGs and 1334 GO terms among the downregulated DEGs (Additional file 6). Among the DEGs, response to stimuli, metabolic processes and biological regulation in biological process and antioxidant activity in molecular function were the obvious enriched GO terms (Fig. 5c). It is noteworthy that expression levels of 17 peroxidase genes were significantly regulated in PpSnRK1α overexpression plants, especially peroxidase 21 was up-regulated 6 times and may improve the ability of transgenic plants to remove ROS under salt conditions (Additional file 4). This hypothesis was supported by the patterns of ROS accumulation in the various lines (Fig. 3).
Antioxidant enzyme activities and the contents of O2− in WT and PpSnRK1α overexpression lines. a Comparison of the O2− content between the WT and PpSnRK1α overexpression lines, b NBT staining for superoxide in the leaves c Comparison of the CAT activity between the WT and PpSnRK1α overexpression lines d DAB staining for H2O2 in the leaves e, f Comparison of the SOD (e) and POD (f) activity between the WT and PpSnRK1α overexpression lines. Note: in a, c, e and f, each sample has three biological replicates. The data are shown as means ± S.E. of three technical repeats
PpSnRK1α overexpression alters the expression of several transcription factors and downstream genes, especially the transcript levels of stress-related genes
Of all the DEGs, we identified 101 transcription factors (TFs) that belong to 37 families (Additional files 7 and 8). Interestingly, the expression levels of TFs from the B3, GARP-G2-like, LOB, and WRKY families were upregulated, whereas those of TFs from other families, such as GRF (growth-regulating factors) and HSF (heat shock transcription factors), were suppressed. Given that over 100 TFs had altered expression levels upon PpSnRK1α overexpression, we concluded that the TFs were directly or indirectly affected by PpSnRK1α, and PpSnRK1α may regulate the expression of some genes or TFs by directly binding to their promoters.
To further investigate the role of SnRK1 in stress response, the expression levels of six stress-responsive genes between the WT and PpSnRK1αoe both two conditions were compared. Of the DEGs, there were genes encoding the stress-responsive protein, including phosphatase 2C (solyc03g096670.3), PYL (pyrabactin resistance(PYR)like) /PYR (pyrabactin resistance)(solyc06g050500.2 and solyc01g095700.3) involved in ABA signaling pathway [32].Surprisingly, the regulated TFs also included salt-related genes, such as NAC (No apical meristem domain-containing protein) family, some members of which have been shown to enhance the tolerance of rice and Arabidopsis to various abiotic stresses. As shown in Fig. 6a, RNA-seq showed that SlPP2C37, a member of the abiotic stress tolerance-related PP2C family, was significantly down-regulated under normal condition, and the expression levels of SlPP2C37 was upregulated in PpSnRK1αoe and WT under salt stress, whereas the expression level of SlPP2C37 was 1.3-fold lower than that in the WT plants (Fig. 6a, b). Furthermore, the expression levels of ABA-receptors SlPYL4 (solyc06g050500.2) and SlPYL8 (solyc01g095700.3) in PpSnRK1α overexpression plants were also altered (Fig. 6c, d, e, f). For example, the SlPYL4 transcript level increased by 11 to 13-fold in PpSnRK1αoe under both normal condition and salt stress, which was consistent with the result of RNA-seq (Fig. 6c, d); whereas PYL8 expression declined in the transgenic lines under normal condition consistent with the result of RNA-seq and slightly increased under salt stress (Fig. 6e, f). Interestingly, SlNAC022 and SlNAC042 were significantly upregulated higher in PpSnRK1αoe than that in WT under both normal condition and salt stress, and the up-regulated expression levels of SlNAC022 and SlNAC042 in the PpSnRK1αoe were 359.1-fold and 52.0-fold, respectively, higher than those in the WT under salt stress (Fig. 6g, h, i, j). These data indicated that the overexpression of SnRK1α could alter the expression of stress-related genes, and had the potential for improving the salt tolerance of tomato plants.
Discussion
When plant responses to salt stress, some protein kinases are activated, which then regulates a large number of defense-related genes, and these changes are conducive to the establishment of defense mechanisms in plants. The response of plants to salt stress is closely related to the SnRKs family, which can be through the SOS (Salt Overly Sensitive) signaling pathway and ABA signal transduction [2]. Although it is unclear how plants respond specifically to stress, there is ample evidence that SnRKs can participate in resistance to salt stress in many species [18, 33, 34].
As a key component of the cell signaling network, SnRK1 can interact with sugar molecules of different forms and energy states, regulate the activities of other kinases, and interact with TFs to maintain energy balance within the cell under stress conditions [18]. To our knowledge, AKIN10(homologous with PpSnRK1α) can negatively regulate the accumulation of AtMYC2 protein via AKIN10 kinase activity-dependent protein modification, thereby responding to abiotic stress [35]. SnRK1 also controls Na+ flux and maintains Na/K homeostasis under high salinity stress [36]. Moreover, SnRK1 can respond to high or low glucose levels in the dark as well as under hypoxia and saline conditions, and regulate energy metabolism in plants under stress [37]. In this study, we identified PpSnRK1α and showed that ectopically expressing it in tomato enhanced salt tolerance phenotypes (Fig. 1). Consistent with previous studies, overexpressing PpSnRK1α lines had higher cell viability and grew better under salt stress, which was accompanied by higher PpSnRK1α expression level and SnRK1 activity (Fig. 1), indicating that SnRK1 is a positive regulator of salt tolerance in tomato. Therefore, PpSnRK1α may have potential additional mechanisms in addition to its role in salt tolerance.
Accumulation of reactive oxygen species is one of the main responses of plants to salt stress. Excessive ROS will destroy biological macromolecules and produce toxic effects on cells. Several antioxidant enzymes in plants, such as antioxidant dismutase, peroxidase, and catalase, play critical roles in the process of scavenging ROS [38]. It was reported that ROS could also be used as a signaling molecule to mediate plant responses to different stresses [39, 40]. Some protein kinases in the cell can sense the stimulation of exogenous ROS or the production of ROS, and respond to stress through a series of phosphorylation and dephosphorylation signals. For example, NADPH oxidase can rapidly increase the intracellular ROS level, and the stimulus signal quickly enters the nucleus from the plasma membrane of the cell [41]. Szymańska, P. Katarzyna et al. found that two SNF1-releted protein kinases 2 (SnRK2), SnRK2.4 and SnRK2.10, could regulate ROS homeostasis and respond to salinity in Arabidopsis [42]. In our investigation, overexpressing PpSnRK1α lines had increased ROS scavenging capacity, low degree of cell membrane damage, and remarkably higher expression of antioxidant enzyme genes under salt stress, which could partially explain the mechanism responsible for the improved salt tolerance of the transgenic plants and suggest a role of PpSnRK1α in regulating the expression of antioxidase genes (Figs. 2, 3, 4, and 7). In general, proline is considered to be one of the osmotic regulators in wheat to resist salt stress, and the accumulation of proline help maintain the integrity and function of the cellular vacuolar membrane, thereby enhancing its ability to resist salt damage [43, 44]. It is apparent that PpSnRK1α helps to maintain the integrity and function of cell membranes, thus enhancing the plant’s ability to resist salt damage (Fig. 2). Therefore, we think that these may have contributed, at least partially, to the observed salt tolerance.
The SnRKs represent a large family that can be further divided into the SnRK1, SnRK2, and SnRK3 subfamilies. Many members of the SnRKs family have been shown to play key roles in abiotic stress responses. AREB1/ABF2, AREB2/ABF4, and ABF3 are activated by SnRK2.2, SnRK2.6 andSnRK2.3 in ABA signaling in response to osmotic stress during vegetative growth [45]. SnRK2.2 and the closely related SnRK2.3 are known to play roles in ABA inhibition of seed germination in Arabidopsis [46]. Additionally, SnRK2.6 can interact with ABAR/CHLH and response to abscisic acid in guard cell signaling [23], The results showed that when plants were under salt stress, the overexpression of PpSnRK1α significantly affected the expression levels of seven SnRK2 family genes in tomato (Fig. 4). Moreover, the research about the SnRKs family revealed that there are also interactions between different genes in the family. For example, in Arabidopsis, SnRK3.15 can interact with SnRK1.1 and SnRK1.2, regulate the activity of them and participate in sugar metabolism [47]. Therefore, the direct or indirect interaction between members of different subfamilies of the plant SnRKs strengthens the plant’s ability to respond to stress.
Abscisic acid is a key player in regulating abiotic stress response in plants. A previous study has evidenced the interaction between SnRK1.1 and ABA signaling—the crossing of an SnRK1.1 overexpression Arabidopsis line with the aba2 mutant, which was impaired in both ABA signaling transduction and sugar signaling pathway, led to glucose hyposensitivity [48]. In addition, defective kernel 33 (DEK33) is involved in the ABA synthesis process and can interact with SnRK1, suggesting that their interaction may regulate the stability of DEK33 and thus the ABA signal [49]. It was reported that inhibition of SnRK1 expression in peas resulted in pleiotropic maturation defects similar to those of the ABA insensitive mutants and SnRK1 can respond to ABA treatment in wheat roots, the level of which drops sharply, but the amount of phosphorylated (active) SnRK1 remains constant [50, 51]. In our study, we found that the expression of SlPP2C37 (a member of PP2C family) [26], SlPYL4, SlPYL8 (an ABA receptor in ABA signaling) and SnRK2 family genes were significantly regulated upon PpSnRK1α overexpression under the normal growth condition as revealed by the RNA-seq data (Figs. 5, 6 and 7). MAPK (mitogen activated protein kinase) cascades have been shown to be implicated in ABA signaling and abiotic stress as well [32]. The RNA-seq results showed that the MAPK pathway was significantly regulated in the PpSnRK1α overexpression lines (Fig. 5, Additional file 4). These genes have been shown to be involved in the ABA signaling pathway [5]. According to these results, we presumed that SnRK1α may be involved in the ABA signal transduction pathway. Further verification is needed to illustrate the regulatory mechanism of SnRK1α under salt stress.
IGV (Integrative Genomics Viewer) visualization of genes by RNA-seq and results of RT-qPCR analysis of related genes a, c, e, g and i IGV visualization of SlPP2C37 (a), SlPYL4 (c), SlPYL8(e), SlNAC022 (g)and SlNAC042(i) b, d, f, h and j Results of RT-qPCR analysis of SlPP2C37 (b), SlPYL4 (d), SlPYL8(f), SlNAC022 (h)and SlNAC042(j). Note: in b, d, f, h and j, error bars indicate SEs (n = 3, 3 biological replicates). All values for WT and PpSnRK1α overexpression plants are statistically significantly different from each other (Student’s t-test; p-value < 0.05)
The working model of PpSnRK1α regulating salt tolerance through the ABA signal transduction pathway and ROS mechanism. In the current working model, PpSnRK1α-overexpression improves the transcription levels of SOD, POD, and CAT1 as well as SOD, POD and CAT activity, reduces ROS production in plant cells. PpSnRK1α is involved in the ABA signaling pathway by altering the transcription levels of ABA receptors (SlPYL4, SlPYL8), PP2C (SlPP2C37) and SlSnRK2s, and thereby leads to an increase in tolerance to salt stress in tomato. Additionally, PpSnRK1α-overexpression increases the expression of SlNAC022, which has been proved to participate in ABA signaling transcription
NAC proteins, as one of the largest family of transcription factors unique to plants, are known to possess diverse roles in plant response to environmental stress [52]. It was reported that ANAC036 (NAC transcription factor family member in Arabidopsis) involved in inflorescence and leaf morphogenesis in Arabidopsis [53]. And Hong Y et al. found that ONAC22 (NAC transcription factor family member in rice) played a positive role in drought and salt stress tolerance through modulating an ABA-mediated pathway [54]. Our investigation showed that SlNAC022, which has high homology with ANAC036 and ONAC22, was significantly regulated in PpSnRK1α overexpressing plants (Fig. 6). Overexpression of JUNGBRUNNEN1 (JUB1) can strongly delay senescence and enhanced tolerance to various abiotic stresses in Arabidopsis [55, 56]. Interestingly, the expression level of SlNAC42, which has high homology with JUB1, is also significantly higher than that in WT plants (Fig. 6). Here we hypothesize that the up-regulation of SlNAC022 and SlNAC42 in PpSnRK1α overexpressing leaves enhances plant salt tolerance in response to ABA signals. More work is required to further determine if SnRK1 and NAC022/ NAC42 interact directly to improve salt tolerance.
Here we showed that over-expression of PpSnRK1α improved the salt tolerance of plants. These observations indicated that stress resistance could be affected by the regulation of the PpSnRK1α gene. Further molecular and genetic analysis will increase our understanding of the function of PpSnRK1α in plant stress resistance and provide strategies for breeding transgenic crop varieties with high-resistance.
Conclusions
In summary, the results of this study indicate that SnRK1 functions as a key kinase for stress response and PpSnRK1α overexpression can significantly improve salt tolerance via regulating ROS metabolism or possibly ABA-mediated pathways. What’s more, SnRK1 may direct or indirect interacts with the SnRK2 family. The mechanism by which SnRK1 responds to salt stress is complex, and whether SnRK1 directly regulates NAC022 and NAC042 expression or indirectly controls their transcription by bind to intermediate receptors warrants further investigations. Generating snrk1 loss-of-function mutants might be necessary to further understand the molecular mechanisms underlying the function of SnRK1 in salt stress response and tolerance.
Methods
Vector construction and tomato transformation
In this study, the WT tomato (Solanum lycopersicum Mill. cv. Zhongshu 6) were kindly provided by Qing-wei Meng [57]. Then we obtained over-expressing PpSnRK1α tomatoes by vector construction and tomato transformation, and selected three lines (OE-1, OE-4, and OE-7) for further research.
Based on the coding sequence of PpSnRK1α (https://www.ncbi.nlm.nih.gov/nuccore/XM_007215174.2), two primers were designed for the amplification (forward primer: 5′- GCTCTAGAATGGATGGATCGGTTG-3′; reverse primer: 5′- GCGTCGACTTAAAGGACCCG − 3′). Then, the PpSnRK1α coding sequence was inserted in the sense orientation into the binary vector pBI121 downstream of the 35S promoter of the cauliflower mosaic virus (35S: PpSnRK1α). The construct was introduced into the Agrobacterium tumefaciens strain LBA4404 for tomato (Solanum lycopersicum Mill. cv. Zhongshu 6) transformation using the leaf disc method [58].
T2 transgenic plants overexpressing PpSnRK1α were identified by the T5 direct PCR kit (TSINGKE, TSE011) using primers PpSnRK1α-DF (5′-GTGTGGAGTTGCGGAGTCAT-3′) and PpSnRK1α-DR (5′-ACGAGGAAGATGAGCCTGGA-3′). The PCR-positive tomato plants were transplanted into pots with soil and grown under natural conditions. Next, we selected three transgenic tomato lines (OE-1, OE-4, and OE-7) for further research.
Plant materials and treatments
The WT and transgenic tomato seeds were cultured for 4 weeks in the greenhouse at 25 ± 3 °C under a 16 h light /8 h dark cycle. After 1 month, the WT and the three transgenic lines, OE1, OE4, and OE7, were treated by either water (the control treatment) or 100 mmol·L− 1 sodium chloride (NaCl). Each treatment contained three groups with ten seedlings per group. Salt stress was introduced by watering the plants with 1000 mL 100 mmol·L-1 NaCl solution until saturation every 3 days. The leaves samples were collected at days 0, 3, 6, 9 and 12 after the treatment. SnRK1 enzyme activity was measured two and 24 h following the NaCl treatment on day one.
RT-PCR
Total RNA was extracted from tomato leaves using Ultrapure RNA Kit (CWBIO, China, CW0581M) and reverse transcribed into cDNA using PrimeScript™ II 1st Strand cDNA Synthesis Kit (Takara, 6210A). The volume of each cDNA pool was adjusted to give the same PCR signal strength for EF1α after 32 cycles. PpSnRK1 and SlSnRK1 homologous segments were selected to design specific primers (PpSlSnRK1α-F and PpSlSnRK1α-R) for PCR identification (Additional file 9). PCR was carried out using the Premix Taq (Takara, R004A).
SnRK1 activity determination
One gram of each fresh plant sample harvested at two and 24 h after the salt treatment, was ground in 1 mL of cold extraction buffer containing 100 mM Tricine-NaOH (pH 8.0), 25 mM NaF, 5 mM dithiothreitol, 2 mM tetrasodium pyrophosphate, 0.5 mM ethylene diamine tetra acetic acid, 0.5 mM ethylene glycol tetra acetic acid, 1 mM benzamidine, 1 mM phenylmethylsulfonyl fluoride, 1 mM protease inhibitor cocktail (Sigma P9599), phosphatase inhibitors (PhosStop; Roche), and insoluble polyvinylpyrrolidone to the solution with a final concentration of 2% (w/v). The homogenate was transferred to two cold microfuge tubes and centrifuged at 12,000×g for 5 min at 4 °C. The supernatant (750 uL) was desalted on a 2.5 mL centrifuge column (Sephadex G-25 medium columns; GE Healthcare) that was pre-equilibrated. SnRKl activity was determined by the Universal Kinase Activity Kit (R&D Systems, Minneapolis, MN, United States, EA004) by using AMARA polypeptide as the substrate [59].
Determination of antioxidant capacity and the content of proline
Proline content was evaluated by the acid ninhydrin method [60]. The O2− content was quantified by the hydroxylamine oxidation method [61]. 3,3′-diaminobenzidine (DAB) staining and nitroblue tetrazolium (NBT) staining were performed to detect H2O2 and O2− levels in tomato leaves. Specifically, tomato leaves were first immersed in a 0.5 mg/mL DAB staining solution (pH = 3.8) or 0.5 mg/ml NBT solution, and then placed in a dark, high-humidity plastic box for about 24 h. Next, the staining solutions were decanted and the leaf samples were incubated with an ethanol/lactic acid/glycerol solution (3:1:1, v:v:v) in a boiling water bath for 5 min [62]. The SOD activity was determined by the photochemical reduction method using nitroblue tetrazolium [63]. The CAT activity was determined by the ultraviolet absorption method and change in the optical density at 240 nm (OD240) of 0.1 within 1 min was defined as one unit of enzyme activity [64]. POD activity was measured by the guaiacol method at OD470, and one unit enzyme activity was defined as 0.1 decease in OD470 per minute [65]. SOD, POD, and CAT activities are shown as U·min− 1·g− 1 (FW).
The degree of damage to the cells was determined by measuring the relative conductivity of tomato leaves. Ten leaf disks (0.8 cm in diameter) from the WT or transgenic lines were submerged in 20 mL distilled water, vacuumed for 30 min, shaken for 3 h at room temperature, and measured for the initial electric conductivity (S1). Subsequently, these leaf samples were boiled for 30 min and cooled down to room temperature to measure the final electric conductivity (S2). The electric conductivity of pure distilled water was used as the blank (S0). The relative electronic conductance (REC) was calculated as \( \mathrm{REC}=\frac{\mathrm{S}1-\mathrm{S}0}{\mathrm{S}2-\mathrm{S}0}\ast 100 \). MDA content was determined by the thiobarbituric acid method [66]. Tomato leaves were stained with the Evans blue staining solution to assess cell viability [67].
RNA-seq
Leaves of WT and three PpSnRK1α overexpressing lines of tomato grown under normal conditions for 2 months were collected. RNA isolation, quality control, library construction, and Illumina Hiseq sequencing were performed by the Wuhan Metware Biotechnology co., LTD.
Bioinformatic analysis
Quality control
Raw reads were removed low-quality reads and reads containing adapters and poly-N from the raw data, then filtered and examined for the sequencing error rate and GC content. The resulting clean, high-quality reads were used for subsequent analyses.
Mapping and differentially expressed gene (DEG) identification
The sequences were mapped to the tomato genome using HISAT2. The FPKM and count value were calculated using feature Counts. DESeq2 was used to analyze the differences between different samples and treatment groups [30, 31]. Then Multiple hypothesis test correction was performed using the Benjamin-Hochberg method at a significance level of P < 0.05; |log 2 fold change| ≥ 1 was used to identify differentially expressed genes (DEGs) between the two libraries (PpSnRK1αoe and WT). The Gene Ontology (GO) and Kyoto encyclopedia of Genes and Genomes (KEGG) enrichment analyses of the DEGs were performed using R based on the hypergeometric distribution.
qRT-PCR
Quantitative Real-Time PCR (qRT-PCR) was carried out using the UltraSYBR mixture (CWBIO, China, CW2601M). The 25 μL reaction mixture contained 0.5 μL of forward primer (10 μM), 0.5 μL reverse primer (10 μM), 5 ng cDNA template, 12.5 μL 2× UltraSYBR mixture, and 9.5 μL water. The conditions used for qRT-PCR were as follows: 95 °C for 10 min, followed by 40 cycles of 95 °C for 10 s, 60 °C for 30 s, and 72 °C for 32 s. The calculation method for qRT-PCR is 2-ΔΔCt with the EF-1α gene as the endogenous control. Each sample had three independent biological replicates. Refer to Additional file 8 Table S6 for primers used for RT-PCR.
Statistical analysis
Statistical analysis was performed using Microsoft Office Excel 2010 and images were processed with Microsoft PowerPoint 2010. Comparison of the mean values was performed by the Duncan multi-range test in SPSS 20.0. P < 0.05 denotes significant differences.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article and its additional files. The coding sequence of PpSnRK1α sequence is available at NCBI (https://www.ncbi.nlm.nih.gov/nuccore/XM_007215174.2).
Abbreviations
- WT:
-
Wild-type
- PpSnRK1α:
-
SNF-related Kinase 1α subunit (Prunus persica)
- PpSnRK1αoe:
-
PpSnRK1α-overexpressing tomatoes lines
- ROS:
-
Reactive oxygen species
- qRT-PCR:
-
quantitative real-time PCR
- ABA:
-
Abscisic acid
- REC:
-
Relative electronic conductance
- DEGs:
-
Differentially expressed genes
- FPKM:
-
Fragments per kilobase of exon per million fragments mapped
- GO:
-
Gene ontology consortium
- KEGG:
-
Kyoto encyclopedia of genes and genomes
- MAPK:
-
Mitogen activated protein kinase
- CIPK:
-
Calcineurin B-like-interacting protein kinase
- ABI1:
-
ABA-insensitive 1
- PP2CA:
-
Protein phosphatase 2C A group
- TFs:
-
Transcription factors
- GRF:
-
Growth-regulating factors
- HSF:
-
Heat shock transcription factors
- PYL/PYR:
-
Pyrabactin resistance(PYR)like/ pyrabactin resistance
- NAC:
-
NAM, ATAF1,2, and CUC2
- SOS:
-
Salt overly sensitive
References
Yang Y, Guo Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018;217(2):523–39.
Zhu J-K. Abiotic stress signaling and responses in plants. Cell. 2016;167(2):313–24.
Munns R. Genes and salt tolerance: bringing them together. New Phytol. 2005;167(3):645–63.
de Zelicourt A, Colcombet J, Hirt H. The role of MAPK modules and ABA during abiotic stress signaling. Trends Plant Sci. 2016;21(8):677–85.
Zhu J-K. Salt and drought stress signal transduction in plants. Annu Rev Plant Biol. 2002;53:247–73.
Fujii H, Zhu J-K. Osmotic stress signaling via protein kinases. Cell Mol Life Sci. 2012;69(19):3165–73.
Manik SMN, Shi S, Mao J, Dong L, Su Y, Wang Q, Liu H. The calcium sensor CBL-CIPK is involved in Plant's response to abiotic stresses. Int J Genomics. 2015;2015:493191.
Banerjee A, Roychoudhury A. WRKY proteins: signaling and regulation of expression during abiotic stress responses. TheScientificWorldJournal. 2015;2015:807560.
Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K. AP2/ERF family transcription factors in plant abiotic stress responses. Biochim Biophys Acta. 2012;1819(2):86–96.
Puranik S, Sahu PP, Srivastava PS, Prasad M. NAC proteins: regulation and role in stress tolerance. Trends Plant Sci. 2012;17(6):369–81.
Rushton DL, Tripathi P, Rabara RC, Lin J, Ringler P, Boken AK, Langum TJ, Smidt L, Boomsma DD, Emme NJ, et al. WRKY transcription factors: key components in abscisic acid signalling. Plant Biotechnol J. 2012;10(1):2–11.
Ariel FD, Manavella PA, Dezar CA, Chan RL. The true story of the HD-zip family. Trends Plant Sci. 2007;12(9):419–26.
Emanuelle S, Hossain MI, Moller IE, Pedersen HL, van de Meene AML, Doblin MS, Koay A, Oakhill JS, Scott JW, Willats WGT, et al. SnRK1 from Arabidopsis thaliana is an atypical AMPK. Plant J. 2015;82(2):183–92.
Crozet P, Jammes F, Valot B, Ambard-Bretteville F, Nessler S, Hodges M, Vidal J, Thomas M. Cross-phosphorylation between Arabidopsis thaliana sucrose nonfermenting 1-related protein kinase 1 (AtSnRK1) and its activating kinase (AtSnAK) determines their catalytic activities. J Biol Chem. 2010;285(16):12071–7.
McCartney RR, Schmidt MC. Regulation of Snf1 kinase: ACTIVATION REQUIRES PHOSPHORYLATION OF THREONINE 210 BY AN UPSTREAM KINASE AS WELL AS a DISTINCT STEP MEDIATED BY THE Snf4 SUBUNIT. J Biol Chem. 2001;276(39):36460–6.
Shen W, Reyes MI, Hanley-Bowdoin L. Arabidopsis protein kinases GRIK1 and GRIK2 specifically activate SnRK1 by phosphorylating its activation loop. Plant Physiol. 2009;150:996–1005.
Stein SC, Woods A, Jones NA, Davison MD, Carling D. The regulation of AMP-activated protein kinase by phosphorylation. Biochem J. 2000;345(3):437.
Baena-González E, Sheen J. Convergent energy and stress signaling. Trends Plant Sci. 2008;13(9):474–82.
Hong S-P, Carlson M. Regulation of Snf1 protein kinase in response to environmental stress. J Biol Chem. 2007;282(23):16838–45.
Cho Y-H, Hong J-W, Kim E-C, Yoo S-D. Regulatory functions of SnRK1 in stress-responsive gene expression and in plant growth and development. Plant Physiol. 2012;158(4):1955.
Soto-Burgos J, Bassham DC. SnRK1 activates autophagy via the TOR signaling pathway in Arabidopsis thaliana. PLoS One. 2017;12(8):e0182591.
Coello P, Hey SJ, Halford NG. The sucrose non-fermenting-1-related (SnRK) family of protein kinases: potential for manipulation to improve stress tolerance and increase yield. J Exp Bot. 2010;62(3):883–93.
Liang S, Lu K, Wu Z, Jiang S-C, Yu Y-T, Bi C, Xin Q, Wang X-F, Zhang D-P. A link between magnesium-chelatase H subunit and sucrose nonfermenting 1 (SNF1)-related protein kinase SnRK2.6/OST1 in Arabidopsis guard cell signalling in response to abscisic acid. J Exp Bot. 2015;66(20):6355–69.
Chen L, Ren F, Zhou L, Wang Q-Q, Zhong H, Li X-B. The Brassica napus Calcineurin B-like 1/CBL-interacting protein kinase 6 (CBL1/CIPK6) component is involved in the plant response to abiotic stress and ABA signalling. J Exp Bot. 2012;63(17):6211–22.
Ohta M, Guo Y, Halfter U, Zhu J-K. A novel domain in the protein kinase SOS2 mediates interaction with the protein phosphatase 2C ABI2. Proc Natl Acad Sci. 2003;100(20):11771.
Rodrigues A, Adamo M, Crozet P, Margalha L, Confraria A, Martinho C, Elias A, Rabissi A, Lumbreras V, González-Guzmán M, et al. ABI1 and PP2CA phosphatases are negative regulators of Snf1-related protein Kinase1 signaling in Arabidopsis. Plant Cell. 2013;25(10):3871.
Yu Y, Fu J, Xu Y, Zhang J, Ren F, Zhao H, Tian S, Guo W, Tu X, Zhao J, et al. Genome re-sequencing reveals the evolutionary history of peach fruit edibility. Nat Commun. 2018;9(1):5404.
Ashraf M, Foolad MR. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ Exp Bot. 2007;59(2):206–16.
Yang Y, Tang N, Xian Z, Li Z. Two SnRK2 protein kinases genes play a negative regulatory role in the osmotic stress response in tomato. Plant Cell Tissue Organ Cult. 2015;122(2):421–34.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550.
Varet H, Brillet-Guéguen L, Coppée J-Y, Dillies M-A. SARTools: a DESeq2- and EdgeR-based R pipeline for comprehensive differential analysis of RNA-Seq data. PLoS One. 2016;11(6):e0157022.
Danquah A, de Zelicourt A, Colcombet J, Hirt H. The role of ABA and MAPK signaling pathways in plant abiotic stress responses. Biotechnol Adv. 2014;32(1):40–52.
Wang P, Xue L, Batelli G, Lee S, Hou Y-J, Van Oosten MJ, Zhang H, Tao WA, Zhu J-K. Quantitative phosphoproteomics identifies SnRK2 protein kinase substrates and reveals the effectors of abscisic acid action. Proc Natl Acad Sci. 2013;110(27):11205.
Li R, Zhang J, Wei J, Wang H, Wang Y, Ma R. Functions and mechanisms of the CBL–CIPK signaling system in plant response to abiotic stress. Prog Nat Sci. 2009;19(6):667–76.
Im JH, Cho Y-H, Kim G-D, Kang G-H, Hong J-W, Yoo S-D. Inverse modulation of the energy sensor Snf1-related protein kinase 1 on hypoxia adaptation and salt stress tolerance in Arabidopsis thaliana. Plant Cell Environ. 2014;37(10):2303–12.
Hwang H-H, Wang C-H, Huang H-W, Chiang C-P, Chi S-F, Huang F-C, Yen HE. Functional analysis of McSnRK1 (SNF1-related protein kinase 1) in regulating Na/K homeostasis in transgenic cultured cells and roots of halophyte Mesembryanthemum crystallinum. Plant Cell Rep. 2019;38(8):915–26.
Polge C, Thomas M. SNF1/AMPK/SnRK1 kinases, global regulators at the heart of energy control? Trends Plant Sci. 2007;12(1):20–8.
You J, Chan Z. ROS Regulation During Abiotic Stress Responses in Crop Plants. Front Plant Sci. 2015;6:1092.
Miller GAD, Suzuki N, Ciftci-Yilmaz S, Mittler RON. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010;33(4):453–67.
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004;9(10):490–8.
Bedard K, Krause K-H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87(1):245–313.
Szymańska PK, Polkowska-Kowalczyk L, Lichocka M, Maszkowska J, Dobrowolska G. SNF1-Related Protein Kinases SnRK2.4 and SnRK2.10 Modulate ROS Homeostasis in Plant Response to Salt Stress. Int J Mol Sci. 2019;20(1):143.
Zhao FG, Qin P. Protective effect of exogenous polyamines on root tonoplast function against salt stress in barley seedlings. Plant Growth Regul. 2004;42(2):97–103.
Liu J, Zhu JK. Proline accumulation and salt-stress-induced gene expression in a salt-hypersensitive mutant of Arabidopsis. Plant Physiol. 1997;114(2):591.
Yoshida T, Fujita Y, Maruyama K, Mogami J, Todaka D, Shinozaki K, Yamaguchi-Shinozaki K. Four Arabidopsis AREB/ABF transcription factors function predominantly in gene expression downstream of SnRK2 kinases in abscisic acid signalling in response to osmotic stress. Plant Cell Environ. 2015;38(1):35–49.
Wang P, Zhu J-K, Lang Z. Nitric oxide suppresses the inhibitory effect of abscisic acid on seed germination by S-nitrosylation of SnRK2 proteins. Plant Signal Behav. 2015;10(6):e1031939.
Yan S, Dong X. Perception of the plant immune signal salicylic acid. Curr Opin Plant Biol. 2014;20:64–8.
Jossier M, Bouly J-P, Meimoun P, Arjmand A, Lessard P, Hawley S, Grahame Hardie D, Thomas M. SnRK1 (SNF1-related kinase 1) has a central role in sugar and ABA signalling in Arabidopsis thaliana. Plant J. 2009;59(2):316–28.
Dai D, Tong H, Cheng L, Peng F, Zhang T, Qi W, Song R. Maize Dek33 encodes a pyrimidine reductase in riboflavin biosynthesis that is essential for oil-body formation and ABA biosynthesis during seed development. J Exp Bot. 2019;70(19):5173–87.
Radchuk R, Radchuk V, Weschke W, Borisjuk L, Weber H. Repressing the expression of the SUCROSE NONFERMENTING-1-RELATED PROTEIN KINASE gene in pea embryo causes pleiotropic defects of maturation similar to an abscisic acid-insensitive phenotype. Plant Physiol. 2006;140(1):263–78.
Coello P, Hirano E, Hey SJ, Muttucumaru N, Martinez-Barajas E, Parry MA, Halford NG. Evidence that abscisic acid promotes degradation of SNF1-related protein kinase (SnRK) 1 in wheat and activation of a putative calcium-dependent SnRK2. J Exp Bot. 2012;63(2):913–24.
Olsen AN, Ernst HA, Leggio LL, Skriver K. NAC transcription factors: structurally distinct, functionally diverse. Trends Plant Sci. 2005;10(2):79–87.
Kato H, Motomura T, Komeda Y, Saito T, Kato A. Overexpression of the NAC transcription factor family gene ANAC036 results in a dwarf phenotype in Arabidopsis thaliana. J Plant Physiol. 2010;167(7):571–7.
Hong Y, Zhang H, Huang L, Li D, Song F. Overexpression of a stress-responsive NAC transcription factor gene ONAC022 improves drought and salt tolerance in Rice. Front Plant Sci. 2016;7:4.
Wu A, Allu AD, Garapati P, Siddiqui H, Dortay H, Zanor M-I, Asensi-Fabado MA, Munné-Bosch S, Antonio C, Tohge T, et al. JUNGBRUNNEN1, a reactive oxygen species–responsive NAC transcription factor, Regulates Longevity in Arabidopsis. Plant Cell. 2012;24(2):482.
Ebrahimian-Motlagh S, Ribone PA, Thirumalaikumar VP, Allu AD, Chan RL, Mueller-Roeber B, Balazadeh S. JUNGBRUNNEN1 Confers Drought Tolerance Downstream of the HD-Zip I Transcription Factor AtHB13. Front Plant Sci. 2017;8:2118.
Meng X, Wang J-R, Wang G-D, Liang X-Q, Li X-D, Meng Q-W. An R2R3-MYB gene, LeAN2, positively regulated the thermo-tolerance in transgenic tomato. J Plant Physiol. 2015;175:1–8.
JEF RBH, Hoffmann NL, Wallroth M, Eichholtz D, Rogers SG, Fraley RT. A simple and general method for transferring genes into plants. Science. 1985;227(4691):1229.
Zhang Y, Primavesi LF, Jhurreea D, Andralojc PJ, Mitchell RA, Powers SJ, Schluepmann H, Delatte T, Wingler A, Paul MJ. Inhibition of SNF1-related protein kinase1 activity and regulation of metabolic pathways by trehalose-6-phosphate. Plant Physiol. 2009;149(4):1860–71.
Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39(1):205–7.
Ma N-N, Zuo Y-Q, Liang X-Q, Yin B, Wang G-D, Meng Q-W. The multiple stress-responsive transcription factor SlNAC1 improves the chilling tolerance of tomato. Physiol Plant. 2013;149(4):474–86.
Hu D-G, Ma Q-J, Sun C-H, Sun M-H, You C-X, Hao Y-J. Overexpression of MdSOS2L1, a CIPK protein kinase, increases the antioxidant metabolites to enhance salt tolerance in apple and tomato. Physiol Plant. 2016;156(2):201–14.
Wang YC, Qu GZ, Li HY, Wu YJ, Wang C, Liu GF, Yang CP. Enhanced salt tolerance of transgenic poplar plants expressing a manganese superoxide dismutase from Tamarix androssowii. Mol Biol Rep. 2009;37(2):1119.
Aebi H. Catalase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. 2nd ed. New York: Academic Press; 1974. p. 673–84.
Pütter J. Peroxidases. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. 2nd ed. New York: Academic Press; 1974. p. 685–90.
Wang Y, Jiang J, Zhao X, Liu G, Yang C, Zhan L. A novel LEA gene from Tamarix androssowii confers drought tolerance in transgenic tobacco. Plant Sci. 2006;171(6):655–62.
Jacyn Baker C, Mock NM. An improved method for monitoring cell death in cell suspension and leaf disc assays using Evans blue. Plant Cell Tissue Organ Cult. 1994;39(1):7–12.
Acknowledgments
We would like to thank the National Natural Science Foundation of China, the National Modern Agroindustry Technology Research System Fund and “Double Tops” Program Foundation of Shandong Province for their financial support.
Funding
This work was supported by The National Modern Agroindustry Technology Research System Fund (No.CARS-30-2-02), National Natural Science Foundation of China (No. 31672099 and 31801812), and “Double Tops” Program Foundation of Shandong Province (SYL2017YSTD10). The funders did not play a role in the experimental design of the study, results analysis or writing of the manuscript, but did provide financial support for the manuscript.
Author information
Authors and Affiliations
Contributions
FP and YX conceived and designed the experiments. WRW and JHL performed the experiments. GFW, MXS, and YSX contributed reagents, materials, and analysis tools. WRW and YSX wrote the article. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1 : Figure S1.
Relative expression level of SnRK1α (the original, uncropped gel).
Additional file 2 : Table S1.
Mapping statistics of RNA-seq reads.
Additional file 3 : Figure S2.
Scatter plot of FPKM values between replicates or genotypes.
Additional file 4 : Table S2.
List of DEGs and their expression levels in WT and PpSnRK1α overexpression lines.
Additional file 5 : Table S3.
KEGG enrichment analysis.
Additional file 6 : Table S4.
GO enrichment analysis.
Additional file 7 : Figure S3.
101 TFs of 37 families regulated by PpSnRK1α.
Additional file 8 : Table S5.
List of TFs and their expression levels in WT and PpSnRK1α overexpression lines.
Additional file 9 : Table S6.
primers used in this study.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Wang, WR., Liang, JH., Wang, GF. et al. Overexpression of PpSnRK1α in tomato enhanced salt tolerance by regulating ABA signaling pathway and reactive oxygen metabolism. BMC Plant Biol 20, 128 (2020). https://doi.org/10.1186/s12870-020-02342-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-020-02342-2