Abstract
Background
Shikonin is a naphthoquinone secondary metabolite with important medicinal value and is found in Lithospermum erythrorhizon. Considering the limited knowledge on the membrane transport mechanism of shikonin, this study investigated such molecular mechanism.
Results
We successfully isolated an ATP-binding cassette protein gene, LeMDR, from L. erythrorhizon. LeMDR is predominantly expressed in L. erythrorhizon roots, where shikonin accumulated. Functional analysis of LeMDR by using the yeast cell expression system revealed that LeMDR is possibly involved in the shikonin efflux transport. The accumulation of shikonin is lower in yeast cells transformed with LeMDR-overexpressing vector than that with empty vector. The transgenic hairy roots of L. erythrorhizon overexpressing LeMDR (MDRO) significantly enhanced shikonin production, whereas the RNA interference of LeMDR (MDRi) displayed a reverse trend. Moreover, the mRNA expression level of LeMDR was up-regulated by treatment with shikonin and shikonin-positive regulators, methyl jasmonate and indole-3-acetic acid. There might be a relationship of mutual regulation between the expression level of LeMDR and shikonin biosynthesis.
Conclusions
Our findings demonstrated the important role of LeMDR in transmembrane transport and biosynthesis of shikonin.
Similar content being viewed by others
Background
Shikonin and its derivatives, which are naphthoquinone pigments synthesized in the roots of the medicinal plant Lithospermum erythrorhizon, possess multiple medicinal and pharmacological properties, such as antibacteria, anti-inflammatory, and antioxidant [1,2,3]. Extensive research has also confirmed the antitumor properties of shikonin and its derivatives [4]. However, the natural resources of L. erythrorhizon have become scarce. In this regard, the two-stage culture system of callus cell culture and hairy roots of L. erythrorhizon has been established as an efficient method for producing useful compounds; in this system, (i) the callus cells or hairy roots are first cultured in a B5 growth medium for rapid proliferation and (ii) transferred into a M9 production medium to efficiently induce the biosynthesis of shikonin and its derivatives [5,6,7,8]. This system also exhibit potential for elucidating the molecular mechanisms of shikonin biosynthesis.
The metabolic pathway of shikonin biosynthesis has been intensively studied. After their biosynthesis in the endoplasmic reticulum, shikonin and its derivatives are postulated to be compartmented in red granules localized in the apoplastic space of cells [6]. The synthesized shikonin and its derivatives are then transported to epidermal cells through a method similar to transport of wax, a lipophilic compound [9]. However, a study reported that the ATP-binding cassette (ABC) transporter (AtWBC12/CER5) also plays an important role in the transmembrane transport of lipophilic compound wax [10]; the Arabidopsis CER5 gene encodes an ABC transporter localized in the plasma membrane of epidermal cells and exports lipid metabolites to the cuticle. Hence, ABC transporters could be involved in transport of shikonin and its derivatives, which are also lipophilic compounds.
The ABC transporter superfamily is one of the largest transporter protein families in plants [11, 12]. Plant ABC transporters possess diverse transport substrates, including lipids, auxin, fatty acids, xenobiotics, heavy metals, and secondary metabolites [13]. Although the transport mechanism of shikonin metabolites remains unknown, several different alkaloid transporters have been reported. The CjMDR transporter is involved in translocation of berberine from the root to the rhizome by transporting it in the plasma membrane of cells around the xylem of the rhizome [14]. The multidrug-resistance protein (MDR), which belongs to the ABCB subfamily, is involved in transport of many divergent compounds [15]. The Nt-JAT transporter unloads nicotine secondary metabolites from the aerial part of a plant to the vacuoles [16]. Hence, other secondary metabolite transporters must be identified.
In our previous transcriptome study, we identified the ABC transporter gene LeMDR, which was significantly up-regulated in cells cultured in M9 production medium compared with that in B5 growth medium. We speculate that the ABC transporter gene of L. erythrorhizon plays an important role in transport of shikonin and its derivatives. To investigate their actual roles in shikonin transport, we cloned the full-length cDNA of LeMDR and analyzed its expression patterns. The function of LeMDR in the transport of shikonin was also investigated using the yeast mutant heterologous expression system. We also analyzed the occurrence of mutual regulation between the expression of LeMDR and shikonin production via overexpression (OE) and RNA interference (RNAi) of LeMDR in the hairy root system of L. erythrorhizon. This study provides new insights to elucidate the transport and biosynthesis regulatory mechanisms of shikonin and its derivatives.
Methods
Plant materials and growth conditions
L. erythrorhizon seeds were stratified in humid sands at 4 °C for approximately 4 weeks. The germinated seeds were grown on soil in growth chambers with 100 μmol·m−2·s−1 light in a 16-h light/8-h dark cycle at 25 °C. For growth under sterile conditions, the seeds were sterilized and grown in culture on half-strength Murashige and Skoog (MS) medium [17]. Ten-month-old L. erythrorhizon seedlings were used to analyze the tissue-specific expression of LeMDR.
Molecular cloning of LeMDR cDNA and bioinformatics analysis
For cloning the full-length cDNA of LeMDR, the rapid amplification of cDNA ends (RACE) strategy was applied using SMART RACE cDNA amplification kit (Clontech, Mountain View, CA, USA). Cell cultures of L. erythrorhizon were harvested for RNA extraction as previously described [18]. First-strand cDNA synthesis was performed according to the manufacturer’s instructions. Gene-specific 5′ and 3′ RACE primers were designed based on the cloned sequence to obtain the full-length fragment (Additional file 1: Table S1).
Clustal W alignments of DNA and protein sequences were conducted with Megalign package (DNAStar, Madison, WI). Protein distance matrix, bootstrap values (1000 replicates), and neighbor-joining consensus trees were calculated using PHYLIP [19]. Arabidopsis thaliana and Coptis japonica sequences were recovered according to the methods proposed by Jasinski et al. [20] and Shitan et al. [21]. The GenBank accession nos. Are as follows: AtMDR1 (AAD31576.1), AtMDR2 (CAB79451.1), AtMDR3 (CAB80675.1), AtMDR4 (AAC34225.1), AtMDR5 (CAB80676.1), AtMDR6 (AAC27839.1), AtMDR7 (BAB10822.1), AtMDR8 (AAG10628.1), AtMDR9 (CAB78807.1), AtMDR10 (AAF17668.1), AtMDR11 (BAB02129.1), AtMDR12 (AAG51476.1), AtMDR13 (BAB02627.1), AtMDR14 (CAB75766.1), AtMDR15 (AAG51482.1), AtMDR16 (AAG10627.1), AtMDR17 (CAB71875.1), AtMDR18 (BAB02852.1), AtMDR19 (BAB02854.1), AtMDR20 (BAB02855.1), AtMDR21 (BAB02858.1), AtMDR22 (BAB02613.1), CjMDR (BAB62040.1).
Genomic southern blot analysis
Southern blot analysis was performed to determine the copy number of the LeMDR gene in the L. erythrorhizon genome. The genomic DNA from the L. erythrorhizon seedling was extracted by using plant genomic DNA extraction kit (TaKaRa Biotech, Japan), and 5 μg of DNA was digested with EcoRI or EcoRV which not only are higher active restriction enzyme but also have no enzymatic sites in the probe sequence of LeMDR for Southern blot. Digested samples were separated on 0.7% agarose gel and transferred in 20 × standard saline citrate (SSC) to a charged nylon membrane (Roche Applied Science, Indianapolis, USA). The membrane was hybridized with a DNA probe encompassing the full-length LeMDR sequences and labeled with DIG-11-dUTP prepared by PCR. Blots were washed 2 × 5 min in 2 × SSC/0.1% SDS at room temperature, then washed 2 × 15 min in 1 × SSC/0.1% SDS at 65 °C and exposed to X-ray film.
Expression analysis of LeMDR
The total RNA from the root, stem, leaf, flower, and rhizome of the ten-month-old intact of L. erythrorhizon was extracted by using TRIzol reagent (Takara Biotech, Japan), and 1 μg of RNA was used to synthesized cDNA by using M-MLV reverse transcriptase (Promega, USA). Real-time PCR was performed with a SYBR Green PCR Master Mix (Toyobo, Japan). The three independent RNA isolates were used for cDNA synthesis, and each cDNA sample was subjected to real-time PCR analysis in triplicate. The glyceraldehyde-3-phosphate dehydrogenase encoding gene (GAPDH), which has been confirmed as a best housekeeping gene in our previous reports [7, 8, 22] was used as a standard and the relative transcript levels were calculated by double delta Ct (ΔΔCt) method.
The EV hairy roots of L. erythrorhizon treated with DMSO (CK), 100 μM shikonin, 20 μM methyl jasmonate (MeJA), and 10 μM indole-3-acetic acid (IAA) for 3 or 8 h were extracted for LeMDR expression pattern analysis. The expression levels of LeMDR were analyzed with CFX manager software (Bio-Rad).
Overexpression, construction of RNAi vectors, and hairy root induction
cDNAs obtained from L. erythrorhizon cells were used directly as PCR template for cloning the full-length cDNA of LeMDR with specific primers [23]. The open reading frame (ORF) of LeMDR was cloned into pBI121 vector [24]. The cassette containing the Cauliflower mosaic virus (CaMV) 35S promoter and LeMDR-eGFP (enhanced green fluorescent protein) was inserted into vectors and a pBI121-LeMDR overexpression (MDRO) construct was obtained.
A specific 390-bp sequence of LeMDR was used for the construction of the LeMDR RNA interference (MDRi) vector. A fragment was generated from L. erythrothizon cDNA by using the PrimeSTAR Max DNA Polymerase (Takara) and specific primers with BamHI and XbaI restriction sites. The resulting PCR product was subcloned into a PCR-blunt vector and subsequently inserted in sense orientation downstream of the GA20 oxidase intron in the pUCC-RNAi vector as described by Chen et al. [25]. The same fragment using specific primers with SpeI and BglII restriction sites was ligated in antisense orientation of pUCC-RNAi already carrying the sense fragment. Finally, the resulting RNAi fragment was excised from pUCC-RNAi using the flanking SpeI/XbaI sites and inserted into the XbaI site of pBI121 vector.
Genetic transformation of Agrobacterium rhizogenes strain ATCC15834 harboring the pBI121-eGFP (EV), pBI121-LeMDR-Overexpression (MDRO), or pBI121-LeMDR-RNAi (MDRi) plasmid were used as infection strains for hairy roots induction. The explants of L. erythrorhizon, including the root, stem, and leaf of sterilized seedling (1 to 1.5 cm), were cut off. The explants were placed on 1/2 MS medium containing 0.2 mg/L 1-naphthaleneacetic acid, 2.0 mg/L 6-benzylaminopurine, and 10 μM acetosyringone and incubated for 1 or 2 days at 25 °C in the dark. Then, the explants were infected with ATCC15834. After 2 to 3 weeks of cultivation, some hairy roots appear.
Subcellular localization of LeMDR in hairy roots and onion epidermis cells
For stable expression, the transgenic hairy roots overexpressing LeMDR were induced as mentioned above and grown on B5 medium for 2 weeks. For transient expression in onion epidermal cells, pBI121-LeMDR-eGFP and pBI121-eGFP were transformed into Agrobacterium strain GV3101. Onion epidermis cell layers were transfected with the constructs pBI121–LeMDR–eGFP and pBI121-eGFP after coating with 0.6-μm gold microparticles using a particle inflow gun as described in Ibrahim et al. [26]. After bombardment at low-pressure helium flow (26 psi), epidermal layers were incubated at room temperature for 16 to 24 h in the dark.
For the detection of eGFP localization, conventional fluorescence microscopy using an Olympus IX-70 microscope with λ ex = 488 nm and λ em = 510 nm was used to screen the hairy roots and onion epidermis cells with eGFP signals.
Functional analysis of LeMDR in yeast cells
LeMDR cDNA (3.9 kb) was subcloned into the yeast expression vector pDR196 [27]. The resulting plasmid, pDR-LeMDR, was used to transform the yeast ABC mutant strain AD12345678 (yor1∆, snq2∆, pdr5∆, pdr10∆, pdr11∆, ycf1∆, pdr3∆, pdr15∆) [28]. The yeast transformant was precultured in 50 mL of SD medium (−uracil), harvested at A 600 = 1.0, and suspended by a 50-mL half-strength SD medium (−uracil) containing 1 mM shikonin, which was dissolved in 0.1% DMSO. The cells were incubated at 30 °C with shaking at 180 rpm, harvested at the indicated times by centrifugation, and washed three times with sterile Milli-Q water [16]. Next, yeast cells were disrupted with acid-washed glass beads in methanol. The samples were centrifuged and supernatants were filtered for high performance liquid chromatography (HPLC) analysis [29].
Measurement of shikonin content in the hairy roots
The MDRO, MDRi, and control (EV) hairy roots were maintained in a growth medium (B5) [30] for 15 days with shaking at 80 rpm at 25 °C. Then, the hairy roots were transferred into M9 production medium [5], maintained on a rotary shaker at 80 rpm, and grown at 25 °C in the dark for 6 days. Both fresh hairy roots and the M9 production medium were extracted with methanol, and the extracts were analyzed by HPLC [29].
Statistical analysis
Statistical analyses were performed using the SPSS 17.0 software (IBM, IL, USA). Student’s t-test was used for comparison between the groups. Error bars indicate the standard deviation for three biological replicates and P < 0.05 (*) or P < 0.01 (**) was considered as statistically significant.
Results
Cloning and sequence analysis of LeMDR
Following differential expression profiling analysis of the transcriptomes between the cells cultured in B5 and M9 media, we discovered a transcript (isotig02082) which was significantly up-regulated in M9 medium (Additional file 2: Figure S1A). We then isolated its full-length cDNA. Blastx analysis indicated that this gene is an ABCB member of the ABC transporter family. In the phylogenetic relationship of plant ABCB transporter, it belonged to the MDR subfamily and was designated as LeMDR (GenBank accession number: KY293693). We then designed the gene-specific primers (Additional file 1: Table S1) and cloned its full-length ORF (Additional file 2: Figure S1B). LeMDR has a typical domain structure containing two nucleotide-binding folds (NBF1 and NBF2). The NBF region contains the highly conserved Walker A and B motifs as well as a sequence known as the ABC signature [15] (Fig. 1a). LeMDR is 3.9 kb in length and encodes putative polypeptides composed of 1296 amino acids and 9 putative transmembrane domains (Additional file 3: Figure S2). LeMDR is most closely related to Coptic japonica CjMDR (Fig. 1b). Given the presumed role of CjMDR involved in the translocation of berberine [21], LeMDR was chosen to verify our assumption that LeMDR protein was possibly involved in the transport of secondary metabolite of L. erythrorhizon.
Molecular characterizations of LeMDR. a ABC motifs in the predicted LeMDR. Alignment of the NBD of LeMDR, A. thaliana MDR1 and MDR2, and C. japonica MDR showing the Walker A and B box and the ABC signature motifs. b Comparative phylogenetic analysis of MDRs from A. thaliana, C. japonica and L. erythrorhizon
The tissue-specific expression analysis in root, stem, leaf, flower, and rhizome suggested that the transcripts of LeMDR dominantly expressed in the root (Fig. 2), where shikonin and its derivatives were synthesized. The genomic southern blot analysis result showed that only one band appeared in each lane in the hybridizations (Additional file 4: Figure S3), indicating that LeMDR genes possibly exist as a single copy in the L. erythrorhizon genome.
LeMDR is localized in the plasma membrane
To investigate the cellular location of LeMDR, the full-length LeMDR cDNA was C-terminally fused to eGFP and expressed under the control of the CaMV 35S promoter in a fusion cassette. We respectively expressed LeMDR-eGFP in the hairy roots and the onion epidermis cells. Confocal microscope analysis of hairy roots (Fig. 3a–b) and heterozygous transgenic onion epidermis cells (Fig. 3c–d) showed green fluorescence signals were predominantly visible in the plasma membrane, whereas epidermal cell transformed with vector control revealed that green fluorescence signals were distributed throughout the cells. Expression of this gene in hairy roots and in onion epidermal cells suggested its plasma membrane localization.
LeMDR functions as a shikonin transporter
To examine the function of LeMDR as a shikonin transporter, LeMDR was expressed with a shuttle vector, pDR196, with which a foreign gene is constitutively expressed by PMA1 promoter [27], in Saccharomyces cerevisiae mutant strain AD12345678. The strain AD12345678 lacks eight major yeast ABC transporter-encoding genes that confer MDR [28]. The same yeast strain transformed with the EV (pDR196) was used as a negative control. Given that LeMDR was suggested to possess export transport activity for shikonin, the time course of shikonin uptake was monitored quantitatively by HPLC analysis. The result showed that the shikonin level in LeMDR-expressing yeast cells is dramatically lower than that in EV yeast cells (Fig. 4).
LeMDR-mediated shikonin transport in yeast cells. Time course analysis of shikonin transport in LeMDR-expressing yeast cells. EV control (pDR196) and LeMDR-expressing (pDR196–LeMDR) yeast cells were incubated in half-strength synthetic dropout medium supplemented with 1.0 mM shikonin. Shikonin was analyzed by HPLC. The error bars represent standard deviations from three biological replicates. Asterisks indicate statistically significant difference compared with control EV (pDR196) (** P < 0.01)
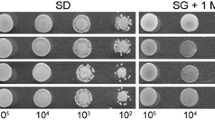
Moreover, we also investigated the substrate specificity by estimating drug sensitivity in yeast transformants. A significant difference in drug sensitivity between LeMDR transformant and the control was found between the 5,8-dihydroxy-naphthoquinone (DNQ) and shikonin, the side chain of the latter cyclized to give an unusual four-membered ring system. The data suggested that shikonin was recognized as a substrate of LeMDR and exported from the LeMDR-expressing yeast vesicles (Additional file 5: Figure S4). However, DNQ accumulated in similar levels as in pDR196-expressing vesicles, indicating that shikonin had relative substrate specificity.
LeMDR affects shikonin biosynthesis in L. erythrorhizon hairy roots
Altering the expression levels of the target gene in the OE and RNAi hairy roots of L. erythrorhizon had been proven to be useful for identifying genes involved in shikonin biosynthesis. Therefore, the overexpression vector MDRO and the RNAi vector MDRi were constructed (Additional file 6: Figure S5A), and pBI121-eGFP empty vector EV was used as control. After infecting with A. rhizogenes 15,834, hairy roots of L. erythrorhizon appeared at the root, stem, and leaf explants (Additional file 6: Figure S5B). The expression level of LeMDR in hairy roots was determined through real-time PCR to confirm the transgenic effects. The results showed that the expression level of LeMDR in the overexpressing hairy roots increased up to four-fold, and RNAi triggered a four-fold decline of transcript levels of LeMDR compared with those found in EV control (Fig. 5a).
Accumulation of shikonin in the hairy roots was analyzed by HPLC. After transgenic hairy root were cultured in B5 liquid medium for 2 weeks, MDRO, EV, and MDRi hairy root lines were transferred to M9 liquid medium in the dark for 6 days. a Expression analysis of LeMDR in MDRO, EV and MDRi hairy roots by real-time PCR, and the housekeeping gene GAPDH was used as control. b HPLC analysis of shikonin content in MDRO, EV and MDRi hairy roots. The error bars represent SD from three biological replicates, and asterisks indicate statistically significant differences of transcript or shikonin levels in MDRO and MDRi compared with EV hairy roots. * P < 0.05, ** P < 0.01
The L. erythrorhizon hairy roots were cultured in hormone-free B5 medium for multiplying growth under light condition. After the hairy roots were transferred to hormone-containing M9 medium in the dark at 24 °C, shikonin and its derivatives were biosynthesized and released to the medium (Additional file 6: Figure S5C), as previously described [31]. The content of shikonin and its derivatives reached the highest level at the sixth day (Fig. 4) [8]. The data of extracted shikonin content indicated that MDRO hairy roots were found to exhibit higher concentration of shikonin than that of EV control, whereas the decline was observed in MDRi hairy roots (Fig. 5b). Basing on the result, we speculated that a significantly positive linear correlation between LeMDR expression level and shikonin production occurred. These results suggested that the biosynthesis of shikonin was also indirectly affected by LeMDR, which was possibly via the efficient transport of the shikonin product out of the hairy root cells by LeMDR.
Expression patterns of LeMDR
To examine the occurrence of mutual regulation on the expression level of LeMDR and shikonin biosynthesis, we analyzed the mRNA expression level of LeMDR in response to the treatment of shikonin, as well as to the treatment of shikonin-positive regulators, MeJA and IAA. Treatment with IAA could significantly increase the biosynthesis of shikonin [32]. MeJA is a specific elicitor for the increased production of shikonin [33] and possesses a stimulating effect on the transcripts of key shikonin biosynthesis-related genes. Treatment of hairy roots with shikonin (Fig. 6a) and IAA (Fig. 6b) significantly increased LeMDR expression by four- and two-fold, respectively, within 8 h. Treatment with MeJA significantly increased the LeMDR expression up to six-fold at 3 h, and two-fold at 8 h (Fig. 6b). These results revealed that the expression of LeMDR was possibly involved in mutual regulation by the product concentration of shikonin.
Relative expression of LeMDR in response to various treatments in the EV hairy roots of L. erythrorhizon by real-time PCR. The hairy roots of L. erythrorhizon were treated for 3 or 8 h with 100 μM shikonin (a) or 20 μM MeJA and 10 μM IAA (b). Results were normalized to GAPDH and are shown relative to the level in DMSO control hairy root (CK). The error bars represent standard deviation from three biological replicates. * P < 0.05, ** P < 0.01
Discussion
ABC transporters represent one of the largest and most conserved protein families in plants [34, 35]. These proteins are ubiquitous and membrane-intrinsic transporters that utilize ATP-binding energy to drive the transport of their substrates across the biological membranes [36]. AtMDR1, the first identified plant ABC transporter protein, was cloned from Arabidopsis thaliana [37]. Subsequent studies have found that plant ABC transporters have diverse transport substrates including hormones [38], lipids [10], heavy metals [39], auxin [40], and xenobiotics [11]. The membrane transport of plant secondary metabolites is a relatively newly developing research area, and ABC transporters have been found to be involved in some plant systems, such as: MATE transporter of Nicotiana tabacum-mediated vacuolar transport of nicotine [16] and CrTPT2 transporter controls leaf surface secretion of anticancer drug components in Catharanthus roseus [41]. However, no ABC transporters responsible for the secondary metabolites of L. erythrorhizon, shikonin and its derivatives, have been reported so far.
In this study, we isolated a LeMDR gene from the shikonin-producing plant L. erythrorhizon. The bioinformatics analysis demonstrated that LeMDR encodes a full ABC protein and possesses the common structural characteristics of all functional MDR-type ABC transporter proteins. The domains analysis showed that LeMDR possessed two transmembrane domains (TMDs) and two nucleotide-binding domains (NBDs), which are arranged in the “TMD1–NBD1–TMD2–NBD2” direction. NBDs have three characteristic motifs common to the ABC transporter family, “Walker A”, “Walker B,” and C motif [42]. The protein is implied to supply the energy to transport the specific substrate by the binding and hydrolysis of ATP.
Our study showed that LeMDR is localized in the plasma membrane of L. erythrorhizon and expressed preferentially in the root of the intact plant where shikonin is synthesized and accumulated [43]. So, the accumulation of shikonin in the roots of L. erythrorhizon cultured in natural soil environment would also be beneficial for the plant because shikonin may protect the underground organs from attack by soil pathogens. The L. erythrorhizon transport system may be a model for understanding such a transport mechanism of shikonin.
MDR-type ABC transporters usually function as drug efflux pumps [21]. When the functionally active LeMDR gene was expressed in yeast, it behaved as an ABC efflux shikonin transporter. LeMDR mediates the transporter of shikonin to the root surface from the biosynthesis site of cells. LeMDR functions presumably actively in the secretion of these endogenous, potentially toxic compounds. Interestingly, the human MATE1 is also a polyspecific exporter that transports nicotine and other toxic compounds [44]. We assume that LeMDR was responsible for unloading of shikonin in the correct orientation because this transport activity was clearly observed in the yeast system.
Hairy root transformation provided an excellent model for the functional analysis of LeMDR. The results showed that LeMDR overexpression under the control of a 35S promoter in hairy root stable transformants strongly affected shikonin biosynthesis. Conversely, LeMDR RNAi decreased the concentration of shikonin in the hairy root lines. The data suggested that LeMDR transporter plays an important role in the accumulation of shikonin possibly by an indirect method, i.e., the efficient transport of shikonin out of the cells by LeMDR would protect the cells from damage and enhance its biosynthesis in turn. Recent study indicates that ABCB4 of Arabidopsis not only mediates efflux of auxin but is also regulated by intracellular auxin concentrations [45]. A characterization of the transport activity of ABCB4 showed that initial IAA accumulation was followed by IAA export [46], and a subsequent study confirmed the substrate-dependent switch to efflux [40]. We speculated that effect on the regulation of LeMDR by the biosynthesis efficiency based on shikonin effective efflux might occur. Given that shikonin could not be effectively transported into the extracellular space, accumulation of metabolites in the intracellular space would inhibit the growth and further production of shikonin [47]. Therefore, the cellular activity and the biosynthesis of shikonin significantly decreased.
Many studies have described successful strategies for the increase in production of secondary metabolites by elicitation techniques [48]. Elicitation is a method for the induction of secondary metabolite that involves the addition of any elicitor, such as MeJA and ethylene, to the culture media [49]. MeJA, as an important plant growth regulator, caused a rapid increase in the activities of enzymes involved in the biosynthesis of shikonin [50]. IAA could significantly increase the biosynthesis of shikonin [32]. If LeMDR confers shikonin transport, a mutual regulation would occur on the expression level of LeMDR by the shikonin product. To examine this postulation, we examined the mRNA expression level of LeMDR in response to the treatment of shikonin, as well as to the treatment of shikonin-positive regulators, MeJA and IAA. Our results showed that the expression of LeMDR was significantly up-regulated by exogenous shikonin. The LeMDR expression of hairy roots treated both with MeJA and IAA indicates that the increase of shikonin production may require more LeMDR for its transport. On the other hand, MeJA and IAA may also directly regulate the expression of LeMDR. These findings are consistent with the hypothesis that an effect of mutual regulation might occur between the expression of LeMDR and shikonin production.
Conclusions
Shikonin, found in L. erythrorhizon, possesses important medicinal value. However, knowledge of its membrane transport mechanism still remains significantly insufficient. In this study, we cloned an ATP-binding cassette protein gene, LeMDR, from L. erythrorhizon and suggested its functions in the membrane transport of shikonin, by using the yeast mutant expression system, as well as its positive regulation on shikonin biosynthesis, by using the hairy root system by the overexpression and RNAi transgenic systems. Our results not only provided the possible theoretical explanation about the role of ABC transporter in shikonin metabolism, but also offer an effective method of increasing the production of secondary metabolites of medicinal plants by genetic engineering.
Abbreviations
- ABC:
-
ATP-binding cassette
- DNQ:
-
5, 8-dihydroxy-naphthoquinone
- eGFP:
-
Enhanced green fluorescent protein
- EV:
-
pBI121-eGFP empty vector
- GAPDH :
-
Glyceraldehydephosphate dehydrogenase gene;
- IAA:
-
Indole-3-acetic acid
- LeMDR :
-
L. erythrorhizon MDR protein gene
- MDR:
-
Multidrug-resistance protein
- MDRi:
-
pBI121-LeMDR-RNAi
- MDRO:
-
pBI121-LeMDR-Overexpression
- MeJA:
-
Methyl jasmonate
- NBD:
-
Nucleotide binding domain
- RACE:
-
Rapid amplification of cDNA ends
- RNAi:
-
RNA interference
- TMD:
-
Transmembrane domain
References
Andújar I, Ríos JL, Giner RM, Recio MC. Pharmacological properties of shikonin - a review of literature since 2002. Planta Med. 2013;79:1685–97.
Ozaki Y, Ohno A, Saito Y, Satake M. Accelerative effect of shikonin, alkannin and acetylshikonin on the proliferation of granulation tissue in rats. Biol Pharm Bull. 1994;17:1075–7.
Papageorgiou VP, Assimopoulou AN, Ballis AC. Alkannins and shikonins: a new class of wound healing agents. Curr Med Chem. 2008;15:3248–67.
Rajasekar S, Park DJ, Park C, Park S, Park YH, Kim ST, et al. In vitro and in vivo anticancer effects of Lithospermum erythrorhizon extract on B16F10 murine melanoma. J Ethnopharmacol. 2012;144:335–45.
Fujita Y, Hara Y, Suga C, Morimoto T. Production of shikonin derivatives by cell suspension cultures of Lithospermum erythrorhizon II - a new medium for the production of shikonin derivatives. Plant Cell Rep. 1981;1:61–3.
Yazaki K, Matsuoka H, Ujihara T, Sato F. Shikonin biosynthesis in Lithospermum erythrorhizon: light-induced negative regulation of secondary metabolism. Plant Biotechnol J. 1999;16:335–42.
Zhao H, Chang Q, Zhang D, Fang R, Zhao H, Wu F, et al. Overexpression of LeMYB1 enhances shikonin formation by up-regulating key shikonin biosynthesis-related genes in Lithospermum erythrorhizon. Biol Plant. 2015;59:429–35.
Fang R, Wu F, Zou A, Zhu Y, Zhao H, Zhao H, et al. Transgenic analysis reveals LeACS-1 as a positive regulator of ethylene-induced shikonin biosynthesis in Lithospermum erythrorhizon hairy roots. Plant Mol Biol. 2016;90:345–58.
Yazaki K. Transporters of secondary metabolites. Curr Opin Plant Biol. 2005;8:301–7.
Pighin JA, Zheng H, Balakshin LJ, Goodman IP, Western TL, Jetter R, et al. Plant cuticular lipid export requires an ABC transporter. Science. 2004;306:702–4.
Rea PA. Plant ATP-binding cassette transporters. Annu Rev Plant Biol. 2007;58:347–75.
Vanholme R, Morreel K, Ralph J, Boerjan W. Lignin engineering. Curr Opin Plant Biol. 2008;11:278–85.
Kaneda M, Schuetz M, Lin B, Chanis C, Hamberger B, Western TL, et al. ABC transporters coordinately expressed during lignification of Arabidopsis stems include a set of ABCBs associated with auxin transport. J Exp Bot. 2011;62:2063–77.
Shitan N, Dalmas F, Dan K, Kato N, Ueda K, Sato F, et al. Characterization of Coptis japonica CjABCB2, an ATP-binding cassette protein involved in alkaloid transport. Phytochemistry. 2013;91:109–16.
Higgins CF. ABC transporters: from microorganisms to man. Annu Rev Cell Biol. 1992;8:67–113.
Morita M, Shitan N, Sawada K, Montagu MCEV, Inzé D, Rischer H, et al. Vacuolar transport of nicotine is mediated by a multidrug and toxic compound extrusion (MATE) transporter in Nicotiana tabacum. Proc Natl Acad Sci U S A. 2009;106:2447–52.
Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–97.
Zhang WJ, Su J, Tan MY, Liu GL, Pang YJ, Shen HG, et al. Expression analysis of shikonin biosynthetic genes in response to M9 medium and light in Lithospermum erythrorhizon cell cultures. Plant Cell Tiss Org. 2010;101:135–42.
Goodman CD, Casati P, Walbot V. A multidrug resistance-associated protein involved in anthocyanin transport in Zea Mays. Plant Cell. 2004;16:1812–26.
Jasinski M, Ducos E, Martinoia E, Boutry M. The ATP-binding cassette transporters: structure, function, and gene family comparison between rice and Arabidopsis. Plant Physiol. 2003;131:1169–77.
Shitan N, Bazin I, Dan K, Obata K, Kigawa K, Ueda K, et al. Involvement of CjMDR1, a plant MDRtype ABC protein, in alkaloid transport in Coptis japonica. Proc Natl Acad Sci U S A. 2003;100:751–6.
Wu SJ, Qi JL, Zhang WJ, Liu SH, Xiao FH, Zhang MS, et al. Nitric oxide regulates shikonin formation in suspension-cultured Onosma Paniculatum cells. Plant Cell Physiol. 2009;50:118–28.
Zhao H, Baloch SK, Kong LR, Zhang WJ, Zou AL, Wang XM, et al. Molecular cloning, characterization, and expression analysis of LeMYB1 from Lithospermum erythrorhizon. Biol Plant. 2014;58:436–44.
Sugikawa Y, Ebihara S, Tsuda K, Niwa Y, Yamazaki K. Transcriptional coactivator MBF1s from Arabidopsis predominantly localize in nucleolus. J Plant Res. 2005;118:431–7.
Chen S, Hofius D, Sonnewald U, Börnke F. Temporal and spatial control of gene silencing in transgenic plants by inducible expression of double-stranded RNA. Plant J. 2003;36:731–40.
Ibrahim M, Siammour A, Celio MR, Mauch F, Menoud P. Construction and application of a microprojectile system for the transfection of organotypic brain slices. J Neurosci Methods. 2000;101:171–9.
Retsch D, Laloi M, Rouhara I, Schmelzer E, Delrot S, Frommer WB. NTR1 encodes a high affinity oligopeptide transporter in Arabidopsis. FEBS Lett. 1995;370:264–8.
Decottignies A, Grant AM, Nichols JW, Wet H, Mclntosh DB, Goffeau A. ATPase and multidrug transport activities of the overexpressed yeast ABC protein Yor1p. J Biol Chem. 1998;273:12612–22.
Albreht A, Vovk I, Simonovska B, Srbinoska M. Identification of shikonin and its ester derivatives from the roots of Echium Italicum L. J Chromatogr A. 2009;1216:3156–62.
Gamborg OL, Miller RA, Ojima K. Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res. 1968;50:151–8.
Brigham LA, Michaels PJ, Flores HE. Cell-specific production and antimicrobial activity of naphthoquincnes in roots of Lithospermum erythrorhizon. Plant Physiol. 1999;119:417–28.
Yazaki K, Kataoka M, Honda G, Severin K, Heide L. cDNA cloning and gene expression of phenylalanine ammonia-lyase in Lithospermum erythrorhizon. Biosci Biotechnol Biochem. 1997;61:1995–2003.
Sakunphueak A, Panichayupakaranant P. Increased production of naphthoquinones in impatiens balsamina root cultures by elicitation with methyl jasmonate. Bioresour Technol. 2010;101:8777–83.
Isenbarger TA, Carr CE, Johnson SS, Finney M, Church GM, Gilbert W, et al. The most conserved genome segments for life detection on earth and other planets. Orig Life Evol Biosph. 2008;38:517–33.
Holland IB. ABC transporters, mechanisms and biology: an overview. Essays Biochem. 2011;50:1–17.
Dean M, Allikmets R. Evolution of ATP-binding cassette transporter genes. Curr Opin Genet Dev. 1995;5:779–85.
Dudler R, Hertig C. Structure of an mdr-like gene from Arabidopsis Thaliana. Evolutionary implications. J Biol Chem. 1992;267:5882–8.
Wu G, Lewis DR, Spalding EP. Mutations in Arabidopsis multidrug resistance-like ABC transporters separate the roles of acropetal and basipetal auxin transport in lateral root development. Plant Cell. 2007;19:1826–37.
Long Y, Li Q, Li J, Cui Z. Molecular analysis, developmental function and heavy metal-induced expression of ABCC5 in zebrafish. Comp Biochem Physiol B Biochem Mol Biol. 2011;158:46–55.
Terasaka K, Blakeslee JJ, Titapiwantanakun B, Peer WA, Bandyopadhyay A, Makam SN, et al. PGP4, an ATP cassette p-glycoprotein, catalyzes auxin transport in Arabidopsis thaliana roots. Plant Cell. 2005;17:2922–39.
Fang Y, Vincenzo DL. ATP-binding cassette transporter controls leaf surface secretion of anticancer drug components in Catharanthus roseus. Proc Natl Acad Sci U S A. 2013;110:15830–5.
Higgins CF, Linton KJ. The ATP switch model for ABC transporters. Nat Struct Mol Biol. 2004;11:918–26.
Yazaki K, Matsuoka H, Shimomura K, Bechthold A, Sato F. A novel dark-inducible protein, LeDI-2, and its involvement in root-specific secondary metabolism in Lithospermum erythrorhizon. Plant Physiol. 2001;125:1831–41.
Otsuka M, Matsumoto T, Morimoto R, Arioka S, Omote H, Moriyama Y. A human transporter protein that mediates the final excretion step for toxic organic cations. Proc Natl Acad Sci U S A. 2005;102:17923–8.
Jenness MK, Murphy AS. Evolution of transport directionality in ABCBs, In: plant ABC transporters; 2014. p. 271–85.
Yang H, Murphy AS. Functional expression and characterization of characterization of Arabidopsis ABCB, AUX 1 and PIN auxin transporters in Schizosaccharomyces pombe. Plant J. 2009;59:179–91.
Deno H, Suga C, Morimoto T, Fujita Y. Production of shikonin derivatives by cell suspension cultures of Lithospermum Erythrorhizon: VI. Production of shikonin derivatives by a two-layer culture containing an organic solvent. Plant Cell Rep. 1987;6:197–9.
Namdeo AG. Plant cell elicitation for production of secondary metabolites: a review. Pharmacogn Rev. 2007;1:69–79.
Krolicka A, Szpitter A, Gilgenast E, Romanik G, Kaminski M, Lojkowska E. Stimulation of anti-bacterial naphthoquinone and flavonoids accumulation in carnivorous plants grown in vitro by addition of elicitors. Enzym Microb Technol. 2008;42:216–21.
Yazaki K, Takada K, Tabata M. Effects of methyl jasmonate on shikonin and dihydroechinofuran production in Lithospermum cell cultures. Plant Cell Physiol. 1997;38:776–82.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC) (31670298, 31470384, 31171161), the Program for Changjiang Scholars and Innovative Research Team in University (IRT_14R27), and the Project of New Century Excellent Talents in University (NCET-11-0234). We thank Dr. Vincenzo De Luca from Brock University (Canada) for the gift of the yeast AD12345678 strain and pDR196.
Availability of data and materials
All supporting data can be found within the manuscript and its additional files.
Author information
Authors and Affiliations
Contributions
Y Zhu, CY Tang, JL Qi, and YH Yang designed research and wrote the paper. Y Zhu, GH Lu, ZW Bian, and FY Wu performed research. Y Zhu, GH Lu, YJ Pang, XM Wang, RW Yang, CY Tang, JL Qi, and YH Yang analyzed data. All authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1: Table S1.
Primer sequences for cloning and expression analysis of the LeMDR gene. (DOC 40 kb)
Additional file 2: Figure S1.
LeMDR cDNA cloning from cell cultures of L. erythrorhizon. (A) Differential expression of the ABC transcript isotig02082 (LeMDR) in the callus cells cultured in B5 and M9 media. (B) The PCR product of the LeMDR ORF. (JPEG 389 kb)
Additional file 3: Figure S2.
Predicted transmembrane helices of LeMDR protein. Four transmembrane domains at the end terminal part. The X-axis represents the LeMDR amino acids position along the protein sequence. (JPEG 566 kb)
Additional file 4: Figure S3.
Southern blot hybridization of L. erythrorhizon with the LeMDR specific probe. The genomic DNA of L. erythrorhizon was digested with various endonucleases. Line 1, positive control: pBI121-LeMDR recombinant plasmid; 2, EcoRI; 3, EcoRV; 4, EcoRI and EcoRV. (JPEG 684 kb)
Additional file 5: Figure S4.
Substrates specificity analysis for LeMDR. Yeast vesicles were prepared from pDR196 or pDR196-LeMDR transformants. After 6 h incubation with shaking, substrate including the shikonin or DNQ accumulated in yeast cells was calculated. The error bars represent standard deviations from three biological replicates. Asterisks indicate statistically significant difference compared with control EV (pDR196). ** P < 0.01. (JPEG 420 kb)
Additional file 6: Figure S5.
Induction and culture of the hairy roots of L. erythrorhizon. (A) Structure of the pBI121-eGFP transformation vectors. (B) Induced the hairy roots with root, stem, and leaf explants. (C) The hairy roots in the B5 liquid medium for multiplication, and the hairy roots in M9 medium for the production of shikonin and its derivatives. (JPEG 1779 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhu, Y., Lu, GH., Bian, ZW. et al. Involvement of LeMDR, an ATP-binding cassette protein gene, in shikonin transport and biosynthesis in Lithospermum erythrorhizon . BMC Plant Biol 17, 198 (2017). https://doi.org/10.1186/s12870-017-1148-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-017-1148-6