Background
In previous studies, we have shown that atypical enteropathogenic Escherichia coli (aEPEC) strains are important diarrheal pathogens among Brazilian children. In the characterization of a collection of 126 aEPEC strains, we identified 29 strains expressing the localized-like adherence (LAL) pattern on HEp-2 cells and harboring large plasmids in the range of 60 to 98 MDa. In this study, we examined 18 of these strains for their ability to transfer the LAL phenotype to a E. coli K-12 C600 strain.
Results
In conjugation experiments, using eight strains which were resistant to one or more antimicrobials and positive for F-pili genes (traA), we were able to cotransfer antimicrobial resistance markers along with adhesion genes. By transforming E. coli DH5α with plasmid DNA from strains A46 (pIS46), A66 (pIS66) and A102 (pIS102), we were able to demonstrate that genes encoding ampicillin, tetracycline and LAL were encoded on a 98-MDa conjugative plasmid. To identify a gene responsible for LAL, we constructed a transposon mutant library of A102 strain. Among 18 mutants that did not adhere to HeLa cells, four carried insertions within fimbrial genes (fimA and traJ) and agglutinin genes (tia and hek). Using these Tn5 mutants as donors, we were able to obtain kanamycin-resistant E. coli MA3456 transconjugants. Sequence analysis of the plasmid genes revealed a region exhibit to 80 and 73% amino acid similarities to the agglutinins Tia and Hek, respectively.
Conclusion
In this study, we have identified three large conjugative plasmids, pIS46, pIS66 and pIS102, coding for antimicrobial resistance and localized-like adherence (LAL) to HeLa cells. In addition, we identified a tia/hek homolog encoded on the pIS102 plasmid, which seems to be involved in adhesion of A102 strain.
Similar content being viewed by others
Background
Enteropathogenic Escherichia coli (EPEC) is a leading cause of infantile diarrhea in developing countries, including Brazil [1,2,3]. EPEC colonizes the small intestine, causing characteristic attaching and effacing (A/E) lesions in the intestinal epithelial cells. The genes necessary for the A/E phenotype are located on a pathogenicity island named the locus of enterocyte effacement (LEE), which encodes a type III secretion system and effectors, the outer membrane adhesin intimin and its translocated receptor (Tir) [4,5,6].
EPEC is divided into typical (tEPEC) and atypical (aEPEC) strains [7, 8]. Typical EPEC strains carry a large virulence plasmid designated the EPEC adherence factor plasmid (pEAF) [9]. The pEAF plasmid encodes the bundle forming pilus (Bfp), which promote bacterium-to-bacterium adherence, resulting in formation of compact microcolonies on the surface of HeLa/HEp-2 cells after 3 h of incubation, a phenotype known as localized adherence (LA) [10, 11]. In contrast, atypical EPEC strains do not posses the pEAF plasmid, and are unable to produce LA. In the absence of Bfp, atypical EPEC strains display a variant LA pattern designated LA-like (LAL) pattern, which is characterized by the presence of compact microcolonies on HEp-2 cells observed after 6 h of infection [12]. LAL is the most common pattern seen among aEPEC strains, however, some strains exhibit diffuse adherence (DA) or aggregative adherence (AA) patterns [13,14,15]. However, not much is known about the adherence factors involved with these phenotypes. In a previous study, we identified two factors contributing to the LAL phenotype of an aEPEC strain E. coli 22 (O26:NM) [16]. A novel afimbrial adhesin called the locus for diffuse adherence, lda, which encodes a diffuse pattern of adherence on HEp-2 cells when cloned into a non-adherent E. coli K12 strain. A second plasmid-encoded factor that contributes to the compact microcolony formation of E. coli 22, but was not characterized.
Atypical EPEC is currently an emerging diarrheal pathogen in both developing and developed countries [17,18,19,20]. In previous studies, we have shown that classic aEPEC strains are important diarrheal pathogens among Brazilian children [21, 22]. In the characterization of a collection of 126 aEPEC strains, we identified 29 strains expressing the LAL pattern on HEp-2 cells [23]. Most of these strains belonged to the classical EPEC serotypes and carried one or two large plasmid bands in the range of 60 to 98 MDa. In this study, we sought to investigate whether these plasmids are involved in the LAL phenotype.
Results
Examination of plasmids in aEPEC LAL+
In this study, we examined 18 aEPEC LAL+ strains harboring large plasmids and belonging to different serotypes for their ability to transfer the LAL phenotype to a E. coli K-12 strain. Strains were characterized for antibiotic resistance to ampicillin (Ap), tetracycline (Tc), chloramphenicol (Cm), kanamycin (Km), and nalidixic acid (Nal), and screened for the presence of conjugal transfer (tra) genes. Among the 18 LAL+ aEPEC strains, 12 were resistant to one or more antimicrobials, and eight of these were positive for F-pili genes (traA) (Table 1).
To test for the presence of LAL plasmids, we perfomed conjugation experiments with the resistant traA-positive strains and the plasmidless non-adherent E. coli K-12 C600 strain. As shown in Table 1, all eight traA-positive strains transferred multiple antibiotic resistance to E. coli C600 strain. The transfer frequencies were low in the range between 10− 6 and 10− 9. Using two transconjugants of each pattern in a HeLa cell adhesion assay we found that only transconjugants from A46 (O55:HND), A66 (O119:HND) and A102 (ONT:HND) strains exhibited the LAL phenotype (Fig. 1).
HeLa cell adherence assay and plasmid profiles of the aEPEC and transconjugant strains. Light microscopy micrographs at 3 h after infection showing the localized-like adherence (LAL) (original magnification, 400x). a aEPEC A46 and transconjugant, b aEPEC A66 and transconjugant, and (c) aEPEC A102 and transconjugant. C600, a non-adherent and plasmidless E. coli K12 strain. MW, 39-R861strain carrying plasmids of known molecular sizes [24]
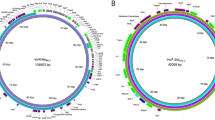
Strain A46 carries two plasmids, one of about 60-MDa and another of 98-MDa, being only the larger plasmid the conjugative. The phenotype of donor strain and transconjugant was Apr Tcr LAL (Fig. 1a). Strain A66 harbors only one large conjugative plasmid of about 98-MDa encoding for Apr Tcr LAL (Fig. 1b). Strain A102 contain only one large conjugative plasmid of about 98-MDa encoding for Apr Tcr LAL (Fig. 1c). Southern blot hybridization with traA genes detected restriction fragments of 2.5-kb in pIS46 and pIS66 plasmids and 1.4-kb in A102 strain, and revealed different profiles (Fig. 2).
In order to confirm the presence of LAL plasmids, the transconjugant plasmids from strains A46 (pIS46), A66 (pIS66), and A102 (pIS102) were transformed into E. coli K-12 DH5α, as described elsewhere [25]. The resulting transformants carrying pIS46, pIS66, or pIS102, were used as donors in a second conjugation experiment with E. coli K-12 J53 as the recipient strain. The seletive plates were minimal medium A [26] containing ampicillin and tetracycline. All Apr Tcr transconjugants showed LAL phenotype.
Nwaneshiudu et al. [27] identified a large conjugative multidrug resistance plasmid in an aEPEC O119:H2 strain MB80. After, we identified variants of the MB80 conjugative resistance in other EPEC strains [28]. In this study, we screened the 18 aEPEC LAL+ strains, and all tested negative for the pMB80 traI and traC PCRs.
Identification of genes involved in LAL phenotype
To identify alternative adhesins to Bfp in aEPEC, we studied strain A102. To identify genes involved in LAL phenotype, we mutagenized strain A102 with the EZ::TN < R6Kγori/KAN-2> Tnp transposome, and screened for adhesion-defective mutants. Among 1100 transposon-inserted mutants screened, 18 mutants that did not adhere to HeLa cells were isolated. All these 18 mutants showed growth rates comparable to those of the parent strains (data not shown). The transposon-inserted locus of each mutant was cloned, as described in Material and Methods, and insertion flanking regions were sequenced. Fourteen transposon insertions were located in genes associated with amino acid metabolism, outer membrane proteins, transcriptional regulators, DNA enzymes, and transport system. Transposon in four mutants were found within fimbrial genes (fimA and traJ) and agglutinin genes (tia and hek) (Table 2).
In this study, we demonstrated that the pIS102 plasmid confers LAL pattern in HeLa cells when expressed in E. coli K-12, and we suspected that the agglutinin genes were located on this plasmid. Thus, we attempted to transfer the agglutinin genes (containing the Tn5-kanamycin) from the mutants II-A-7 and II-F-5 into a nalidixic acid-resistant plasmidless E. coli MA3456. Indeed, we were able to obtain kanamycin-resistant E. coli MA3456 transconjugants using the Tn5 mutant II-A-7 as donor. Plasmid genes flanking the Tn5-transposon insertion were cloned, and a 1200 bp EcoRI fragment containing Tn5 was subjected to DNA sequence. BLAST analysis identified a protein of 123 aminoacids with 80% identity to Tia and 73% identity to Hek (Fig. 3; see Figure S1 in the supplemental material).
Discussion
The frequency of isolation of aEPEC from diarrheal cases has significantly increased in Brazil in the last years [1,2,3]. Atypical EPEC strains lack the EAF plasmid and hence are Bfp negative, but they can still adhere to HEp-2 cells in a Bfp independent LA pattern, which is referred to as LAL [12]. The LAL pattern is characterized by the presence of compact microcolonies on HEp-2 cells, but only after 6 h of incubation, whereas LA is apparent after 3 h.
In the search for potential adherence factors, we studied 18 atypical aEPEC LAL+ strains isolated in a case control study in Brazil [23]. All of the 18 strains harbor one or two large plasmids and many genes encoding for known E. coli adhesins, but to date, no adhesive structure has been implicated in the microcolonies formation of aEPEC [23].
Our results demonstrate a high rate of resistance to certain antimicrobial agents in 12 aEPEC LAL+ strains in which resistance is apparently associated with conjugative plasmids. In addition, we also found plasmids encoding multiple drug resistance along with adhesion genes in three aEPEC strains. By transforming E. coli DH5α with plasmids from A46, A66 and A102 strains, we were able to demonstrate that genes encoding ampicillin and tetracycline resistance and LAL were encoded on a 98-MDa conjugative plasmid. Although an aEPEC conjugative multiresistance plasmid has been described [27], a plasmid coding for antibiotic resistance along with adhesion genes has not been previously reported in aEPEC.
In this study, we used Tn5-based transposon mutagenesis to identify the genetic determinants of aEPEC A102 strain responsible for the LAL phenotype. We have found that multiple factors, such as amino acid metabolism, transcriptional regulators, or transport systems may affect the adherence of A102 strain to HeLa cells. However, we identified mutated genes associated with fimbrial adhesins (fimA and traJ) and agglutinin genes (tia and hek), which suggest a role of these genes in adherence.
The fimbrial gene fimA encodes the larger subunit of type 1 pilus (T1P). T1P has been reported to be responsible for the initial adherence of E. coli K-12 to abiotic surfaces [29], and is an important virulence factor in uropathogenic E. coli strains [30, 31]. However, T1P of prototype typical EPEC strains E2348/69 and B171 had no effect on LA pattern [32] but were important for development of the AA pattern on EAEC strain 042 [33]. The traJ gene regulates the expression of F pilus involved in bacterial conjugation mediated by F plasmids [34]. It has been shown that putative F pilus may work as central adhesion factor during the biofilm formation by typical EAEC strains. In addition, F pili expressed by EAEC strains boosted mixed biofilm formation when in the presence of aggregative Citrobacter freundii [35].
The outer membrane invasin and adhesin Tia previously described in enterotoxigenic E. coli mediates bacterial attachment to a variety of cultured human epithelial cells, autoaggregation, and biofilm formation [36]. The Hek outer membrane protein of E. coli is an auto-aggregating adhesin and invasin reported from uropathogenic E. coli and neonatal meningitic E. coli [37, 38]. Interestingly, agglutinin genes are common in aggregative E. coli (EAEC). EAEC strains harbor the agglutinin genes hra1 (hek), hra2 and tia which confer autoaggregation, biofilm formation, and AA patterns [39, 40]. In this study, we were able to demonstrate that these agglutinin genes were encoded on the pIS102 plasmid. By using the Tn5 mutants as donors, we were able to obtain kanamycin-resistant E. coli MA3456 transconjugants. Sequence analysis of the plasmid genes revealed a region exhibit to 80 and 73% amino acid similarities to the agglutinins Tia and Hek, respectively. Future efforts will be directed to construct a tia/kek homolog deletion mutant of A102 strain.
Conclusions
In this study, we have identified three large conjugative plasmids, pIS46, pIS66 and pIS102, coding for antimicrobial resistance and localized-like adherence (LAL) to HeLa cells. In addition, we identified a tia/hek homolog encoded on the pIS102 plasmid, which seems to be involved in adhesion of A102 strain. Characterization of this tia/hek homolog may bring new insights into aEPEC colonization.
Methods
Bacterial strains
Eighteen aEPEC strains showing LAL and belonging to different serotypes were studied [23]. All strains were isolated from children with diarrhea. The E. coli K-12 strains C600, J53 and DHα5, and E. coli MA3456 were used as the recipient for EPEC plasmids during conjugation or transformation experiments. E. coli DH5αλpir was used for cloning studies. The strains were cultured in Luria-Bertani (LB) broth or on LB agar at 37 °C with appropriate antibiotics when necessary.
Antimicrobial susceptibility testing
Antimicrobial susceptibility tests were performed by the disk diffusion method [41] using disks containing ampicillin (10 μg), chloramphenicol (30 μg), kanamycin (30 μg), nalidixic acid (30 μg), sulfonamide (300 μg), streptomycin (10 μg), and tetracycline (30 μg). The inhibition zone diameters were interpreted according to Clinical and Laboratory Standards Institute requirements [41], and the E. coli NCTC10418 was used as the control.
HeLa cell adherence assay
The HeLa cell adhesion assay was performed as previously described [12] with modifications. Briefly, monolayers of 105 HeLa cells were grown in Dulbecco modified Eagle medium containing 10% fetal bovine serum, by use of 24-well tissue culture plates. Bacteria strains were grown statically in 2 ml of LB for 16–18 h at 37 °C. Cell monolayers were infected with 20 μl of bacterial cultures added to 1 ml of DMEM and were incubated at 37 °C for 3 h. After incubation, the cells were washed with sterile PBS, fixed with methanol, stained with Giemsa stain, and examined under a light microscope.
Plasmid profiling
Plasmid DNA was extracted from overnight bacterial cultures by the alcaline extraction method of Birnboim and Doly [42], and analyzed in 0.8% agarose gels stained with ethidium bromide (5 μg/ml). Plasmid molecular sizes were calculated based on relative migration of plasmids with known sizes contained in strain 39-R861 [24].
DNA hybridization
Colony and Southern blot hybridization [25] were performed at 65 °C. Gene probes, traA [27] were generated by PCR, gel purified, and labeled with [α-32P] dCTP using a Rediprime (Amersham Pharmacia Biotech Inc., EUA) according to the manufacturer-s instructions.
Conjugation experiments
The donor and recipient strains were grown on LB broth to an optical density at 600 nm (OD600) of 0.5 mixed equally, and then inoculated on filter papers for 4 h. The filter paper mixtures were then suspended in LB medium, and dilutions were plated on LB agar containing nalidixic acid (50 μg/ml) with either ampicillin (100 μg), kanamycin (50 μg), chloramphenicol (50 μg/ml) or tetracycline (25 μg/ml). Conjugation frequencies were calculated as the ratio of number of transconjugant colonies by the number of donor colonies. Each conjugation experiment was repeated at least twice. The transconjugants were tested for HeLa cell adherence assay as described previously [12].
Transposon mutagenesis and genetic analysis
Transposon mutants were generated with the kanamycin resistance (Kmr)-encoding transposome EZ::TN < R6Kγori/KAN-2> Tnp transposome (Epicentre Biotechnologies) by electroporation according to the manufacturer’s procedures. Briefly, electrocompetent bacterial cells were transformed with 1 μl of the Tnp transposome. Transposon-inserted bacterial colonies that grew on LB agar plates containing nalidixic acid (100 μg/ml), ampicillin (100 μg/ml) and kanamycin (50 μg/ml) were screened for their adhesion phenotype to HeLa cells as described below. Genomic DNA was isolated from mutants by using the PureLink Genomic DNA Mini kit (Invitrogen). Genomic DNA of the mutants, was digested with EcoRI, self-ligated by the addition of T4 DNA ligase, and then used for transformation of E. coli DH5αλpir. Rescued DNA plasmids were purified by using the Mini plasmid kit (Qiagen) and sequenced by using transposon-specific primers R6KAN-2 RP-1 and KAN-2 FP-1 (Epicentre). DNA sequencing was performed at the Centro de Estudos do Genoma Humano-USP, São Paulo. Nucleotide sequence data were analyzed using SeqMan and MegAlign software and the BLAST tool (http://www.ncbi.nlm.nih.gov/BLAST).
Availability of data and materials
The data is available upon request. Please contact the corresponding author Isabel C A Scaletsky, E-mail: scaletskyunifesp@gmail.com
Abbreviations
- EPEC:
-
Enteropathogenic Escherichia coli
- tEPEC:
-
typical EPEC
- aEPEC:
-
atypical EPEC
- LA:
-
Localized adherence
- LAL:
-
Localized adherence-like
- DA:
-
Diffuse adherence
- AA:
-
Aggregative adherence
- EAF:
-
E. coli adherence factor plasmid
- BFP:
-
Bundle-forming pilus
- TIP:
-
Type 1 pili
References
Nataro JP, Kaper JB. Diarrheagenic Escherichia coli. Clin Microbiol Rev. 1998;11:142–201.
Ochoa TJ, Contreras CA. Enteropthogenic Escherichia coli infection in children. Curr Opin Infect Dis. 2011;24:478–83.
Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the global enteric multicenter study, GEMS): a prospective, case-control study. Lancet. 2013;382:209–22.
Moon HW, Whipp SC, Argenzio RA, Levine MM, Gianella RA. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect Immun. 1983;41:1340–51.
Jarvis KG, Girón JA, Jerse AE, McDaniel TK, Donnenberg MS, Kaper JB. Enteropathogenic Escherichia coli contains a putative type III secretion system necessary for the export of proteins involved in attaching and effacing lesion formation. Proc Natl Acad Sci U S A. 1995;92:7996–8000.
McDaniel TK, Kaper JB. A cloned pathogenicity island from enteropathogenic Escherichia coli confers the attaching and effacing phenotype on E. coli K12. Mol Microbiol. 1997;23:399–407.
Kaper JB. Defining EPEC. Rev Microbiol. 1996;27:130–3.
Trabulsi LR, Keller R, Gomes TAT. Typical and atypical enteropathogenic Escherichia coli. Emerg Infect Dis. 2002;8:508–13.
Baldini MM, Kaper JB, Levine MM, Candy DC, Moon HW. Plasmid-mediated adhesion in enteropathogenic Escherichia coli. J Pediatr Gastroenterol Nutr. 1983;2:534–9.
Donnenberg MS, Girón JA, Nataro JP, Kaper JB. A plasmid-encoded type IV fimbrial gene of enteropathogenic Escherichia coli associated with localized adherence. Mol Microbiol. 1992;6:3427–37.
Scaletsky ICA, Silva MLM, Trabulsi LR. Distinctive patterns of adherence of enteropathogenic Escherichia coli to HeLa cells. Infect Immun. 1984;45:534–6.
Scaletsky ICA, Pelayo JS, Giraldi R, Rodrigues J, Pedroso MZ, Trabulsi LR. EPEC adherence to HEp-2 cells. Rev Microbiol. 1996;27:58–62.
Scaletsky ICA, Pedroso MZ, Oliva CAG, Carvalho RLB, Morais MB, Fagundes-Neto U. A localized adherence-like pattern as a second pattern of adherence of classic enteropathogenic Escherichia coli to HEp-2 cells that is associated with infantile diarrhea. Infect Immun. 1999;67:3410–5.
Abe CM, Trabulsi LR, Blanco J, Blanco M, Dahbi G, Blanco JE, Mora A, Franzolin MR, Taddei CR, Martinez MB, et al. Virulence features of atypical enteropathogenic Escherichia coli identified by the eae+ EAF-negative stx- genetic profile. Diagn Microbiol Infect Dis. 2009;64:357–65.
Tennant SM, Tauschek M, Azzopardi K, Bigham A, Bennette-Wood V, Hartland EL, et al. Characterization of atypical enteropathogenic E. coli strains of clinical origin. BMC Microbiol. 2009;2009(3):9–117.
Scaletsky ICA, Michalski J, Torres AG, Dulguer MV, Kaper JB. Identification and characterization of the locus for diffuse adherence, which encodes a novel afimbrial adhesion found in atypical enteropathogenic Escherichia coli. Infect Immun. 2005;73:4753–65.
Robins-Browne Afset JE, Bevanger L, Romundstad P. Bergh, K: association of atypical enteropathogenic Escherichia coli (EPEC) in prolonged diarrhea. J Med Microbiol. 2004;53:1137–44.
Alikhani MY, Mirsalehian A, Aslani MM. Detection of typical and atypical enteropathogenic Escherichia coli (EPEC) in Iranian children with and without diarrhoea. J Med Microbiol. 2006;146:54–61.
Robins-Browne RM, Bordun A, Tauschek M, Bennett-Wood TM, Russell J, Oppedisano F, et al. Escherichia coli and community-acquired gastroenteritis, Melbourne, Australia. Emerg Infect Dis. 2004;101:1797–805.
Nguyen RN, Taylor L, Tauschek M, Robins-Browne RM. Atypical enteropathogenic Escherichia coli infection and prolonged diarrhea in children. Emerg Infect Dis. 2006;12:597–603.
Araújo JM, Tabarelli GF, Aranda KR, Fabbricotti SH, Fagundes-Neto U, Scaletsky ICA. Typical enteroaggregative and atypical enteropathogenic types of Escherichia coli are the most prevalent diarrhea-associated pathotypes among Brazilian children. J Clin Microbiol. 2007;45:3396–9.
Scaletsky ICA, Aranda KR, Souza TB, Silva NP, Morais MB. Evidence of pathogenic subgroups among atypical enteropathogenic Escherichia coli strains. J Clin Microbiol. 2009;47:3756–9.
Scaletsky IC, Aranda KR, Souza TB, Silva NP. Adherence factors in atypical enteropathogenic Escherichia coli strains expressing the localized adherence-like pattern in HEp-2 cells. J Clin Microbiol. 2010a;48:302–6.
Threlfall EJ, Rowe B, Ferguson JL, Ward LR. Characterization of plasmids conferring resistance to gentamicin and apramycin in strains of Salmonella typhimurium phage type 204c isolated in Britain. J Hyg (Lond). 1986;97:419–26.
Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989.
Davis BD, Mingioli ES. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950;60:17–28.
Nwaneshiudu AI, Mucci T, Pickard DJ, Okeke IN. A second large plasmid encodes conjugative transfer and antimicrobial resistance in O119:H2 and some typical O111 enteropathogenic Escherichia coli strains. J Bacteriol. 2007;189:6074–9.
Scaletsky ICA, Souza TB, Aranda KRS, Okeke IN. Genetic elements associated with antimicrobial resistance in enteropathogenic Escherichia coli (EPEC) from Brazil. BMC Microbiol. 2010b;10:25.
Pratt LA, Kolter R. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type 1 pili. Mol Microbiol. 1998;30:285–93.
Johnson JR. Virulence factors in Escherichia coli urinary tract infection. Clin Microbiol Rev. 1991;4:80–128.
Connell I, Agace W, Klenm P, Schembri M, Marild S, Svanborg C. Type 1 fimbria expression enhances Escherichia coli virulence for the urinary tract. Proc Natl Acad Sci U S A. 1996;93:9827–32.
Elliott S, Kaper JB. Role of type 1 fimbriae in EPEC infections. Microb Pathol. 1997;23:113–8.
Moreira CG, Carneiro SM, Nataro JP, Trabulsi LR, Elias WP. Role of type 1 fimbrae in the aggregative adhesion pattern o enteroaggregative Escherchia coli. FEMS Microbiol Lett. 2003;226:79–85.
May T, Okabe S. Escherichia coli harboring a natural IncF conugative F plasmid develops complex mature biofilms by stimulating synthesis of colanic and curli. J Bacteriol. 2008;190:7479–90.
Pereira AL, Silva TN, Gomes ACMM, Araújo ACG, Giugliano LG. Diarrhea-associated biofilm formed by enteroaggregative Escherichia coli and aggregative Citrobacter freundii: a consortium mediated by putative F pili. BMC Microbiol. 2010;10:57.
Fleckenstein JM, Kopecko DJ, Warren RI. Elsinghorst: molecular characterization of the tia invasion locus from enterotoxigenic Escherichia coli. Infect Immun. 1996;64:2256–65.
Fagan RP, Lambert MA, Smith SG. The Hek outer membrane protein of Escherichia coli strain R218 binds to proteoglycan and utilizes a single extracelular loop for adherence, invasion and autoaggregation. Infect Immun. 2008;76:1135–42.
Srinivasan U, Foxman B, Marrs CF. Identification of a gene encoding heat-resistant agglutinin in Escherichia coli as a putative virulence factor in urinary tract infection. J Clin Microbiol. 2003;41:285–9.
Bhargava S, Johnson BB, Hwang J, Harris TA, George AS, Muir A, Dorff J, Okeke IN. The heat-resistant agglutinin 1 is an acessory enteroaggregative Escherichia coli colonization fator. J Bacteriol. 2009;191:4934–42.
Mancini J, Weckselblatt B, Chung YK, Durante JC, Andelman S, Glaubman J, Dorff JD, Bhargava S, Lijek RS, Unger KP, Okeke IN. The resistant agglutinin family includes a novel adhesin from enteroaggregative Escherichia coli strain 60A. J Bacteriol. 2011;193:4813–20.
National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility tests: 8th edition. Villanova: National Committee for Clinical Laboratory Standards; 2003.
Birnboim HC, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleid Acids Res. 1979;7:1513–23.
Acknowledgments
This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
SSS performed the genetic experiments and MVM analyzed data and contributed to the preparation of the manuscript. ICAS designed the experimental procedures, supervised all the experimental works and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the ethics committee of the Universidade Federal de São Paulo, Brazil. Stool samples were obtained with the written informed consent from the parents or guardians of the children.
Consent for publication
Not Applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1
: Figure S1 BLAST results of the 1175 bp sequence obtained from transconjugant II-A-7. Description of data: Descriptions and alignments of the 1175 pb sequence.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Silva, S.S., Monfardini, M.V. & Scaletsky, I.C.A. Large plasmids encoding antibiotic resistance and localized-like adherence in atypical enteropathogenic Escherichia coli strains. BMC Microbiol 20, 138 (2020). https://doi.org/10.1186/s12866-020-01809-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12866-020-01809-4