Abstract
Background
Control of Mycobacterium tuberculosis (Mtb) infection requires CD4+ T-cell responses and major histocompatibility complex class II (MHC II) presentation of Mtb antigens (Ags). Dendritic cells (DCs) are the most potent of the Ag-presenting cells and are central to the initiation of T-cell immune responses. Much research has indicated that DCs play an important role in anti-mycobacterial immune responses at early infection time points, but the kinetics of Ag presentation by these cells during these events are incompletely understood.
Results
In the present study, we evaluated in vivo dynamics of early Ag presentation by murine lymph-node (LN) DCs in response to Mycobacterium bovis bacillus Calmette–Guérin (BCG) Ag85A protein. Results showed that the early Ag-presenting activity of murine DCs induced by M. bovis BCG Ag85A protein in vivo was transient, appearing at 4 h and being barely detectable at 72 h. The transcription levels of CIITA, MHC II and the expression of MHC II molecule on the cell surface increased following BCG infection. Moreover, BCG was found to survive within the inguinal LN DC pool, representing a continuing source of mycobacterial Ag85A protein, with which LN DCs formed Ag85A peptide-MHCII complexes in vivo.
Conclusions
Our results demonstrate that a decrease in Ag85A peptide production as a result of the inhibition of Ag processing to is largely responsible for the short duration of Ag presentation by LN DCs during BCG infection in vivo.
Similar content being viewed by others
Background
Tuberculosis (TB), caused by infection with Mycobacterium tuberculosis (Mtb), remains a major disease worldwide and is the leading infectious disease in terms of mortality, being responsible for an estimated 1.3 million deaths globally in 2016. Moreover, in the same year, there were an estimated 10.4 million new cases of active TB worldwide. Mycobacterium bovis bacillus Calmette–Guérin (BCG) is the only TB vaccine for humans in current use, but its efficacy is insufficient to prevent pulmonary TB in adults and reactivation of latent Mtb infection [1]. BCG vaccination mainly induces effector, rather than central, memory T cells, which are maintained for a shorter period, explaining the limited duration of protection afforded [2, 3].
CD4+ T-cell responses and the production of interferon gamma (IFN-γ) are particularly important to the containment of Mtb infection [4, 5]. Dendritic cells (DCs) represent the bridge between the innate and adaptive immune responses and specifically strengthen the cellular immune response against mycobacterial infections [6, 7]. Thus, the mechanisms involved in major histocompatibility complex class II (MHC II) antigen (Ag) processing and presentation, which are required for CD4+ T-cell activation, are crucial for controlling Mtb infection [8]. Much research has indicated that DCs play an important role in anti-mycobacterial immune responses in the early stages of infection, but little is known of the kinetics of Ag presentation by these cells soon after M. bovis BCG exposure. Indeed, efforts to understand the basis of protective immunity against Mtb have led us the examinntion of even earlier infection time points. We previously investigated the Ag-presenting cell (APC) functions of murine DCs during the first 2 weeks following intravenous administration of recombinant BCG (rBCG) expressing the Escherichia coli MalE protein as a reporter Ag [9]. However, this process has not yet been directly examined in lymph node (LN) DCs using an endogenous M. bovis BCG Ag.
In the present study, we evaluated the in vivo dynamics of early Ag presentation by murine inguinal LN DCs in response to M. bovis BCG. The results showed that the early Ag-presenting activity of murine DCs induced by M. bovis BCG Ag85A protein in vivo was transient and that the inhibition of Ag processing due to the decreased production of Ag85A peptide is the primary reason for the rapid loss of Ag85A peptide-MHC II complexes.
Results
Stimulation of Ag85A-specific IFN-γ production in BCG-infected mice
In order to evaluate the kinetics of the Ag85A-specific T-cell immune response to BCG infection, mononuclear cells isolated from BCG-immunized mice were stimulated in vitro with Ag85A peptide, Ag85A protein, or bovine purified protein (PPD), and concentrations of IFN-γ in culture supernatants were measured. The result showed a significant increase in Ag85A-specific IFN-γ production by inguinal LN mononuclear cells 3 days after BCG injection, with an even greater increase after 6 days (Fig. 1). Ag85A-specific T lymphocytes in both the spleen (Fig. 1a) and inguinal LN (Fig. 1b) produced high levels of IFN-γ when stimulated with Ag85A, although IFN-γ production was 10-fold higher in the LN group. This suggests that the Ag85A-specific T-cell immune response was initiated in the inguinal LN 6 days following BCG infection. Differences in IFN-γ production in the murine spleen and LN may be a consequence of differences in the frequency of T cells among mononuclear cells.
Detection of IFN-γ production following BCG infection. Four groups of C57BL/6 mice (n = 6) s.c. vaccinated with 1 × 108 CFU BCG were sacrificed at different time points, and their spleens and inguinal LNs were removed. Increased IFN-γ levels were detected in the culture supernatants of splenocytes (a) and inguinal LN cells (b). Results are representative of three independent experiments and presented as means ± SEM. Statistical significance was determined using Student’s t-test (*P < 0.05, **P < 0.01, ***P < 0.001). CM, culture medium
Dynamics of DC ag-presenting activity in vivo
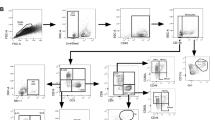
To investigate the dynamics of inguinal LN DC Ag presentation, we tested their capacity to stimulate DE10 T-cell hybridomas at several time points after subcutaneous injection of mice with BCG. The inguinal LN DCs (CD11chigh) were sorted by autoMACS with a purity of 94.7% (Fig. 2a). When mice were infected with BCG, LN DCs collected at early time points invoked a response from DE10 hybridomas, with IL-2 being detected following stimulation with those harvested 4 h post-injection, and the highest IL-2 production being observed in response to DCs from mice infected for 12 h. However, IL-2 was only minimally produced in response to DCs from mice infected for 72 h (Fig. 2b). Interestingly, when mice were s.c. injected with heat-killed BCG, Ag-presenting activity markedly decreased from 12 h to 96 h post-injection, suggesting that live BCG is necessary for efficient Ag presentation by DCs in vivo (Fig. 2c). Together, these results indicate that the MHC II presentation of mycobacteria-derived peptides by inguinal LN DCs is only transient, with Ag85A peptide-MHC II complexes on the surfaces of inguinal LN DCs disappearing rapidly.
Detection of murine LN DCs Ag-presenting activity ex vivo. Suspensions of inguinal LN cells from C57BL/6 mice were stained with anti-CD11c MicroBeads and separated by autoMACS, resulting in a population of 94.7% CD11c+ cells (a). To investigate the dynamics of LN DC Ag-presenting activity, we harvested and sorted LN DCs from the inguinal LN at various time points groups after s.c. injection of mice (n = 6) with BCG or heat-killed BCG. Then, these cells were serially diluted and used to directly stimulate DE10 T-cell hybridomas. In vivo formation of Ag85A peptide-MHC complexes on LN DCs from mice injected with BCG (b) or heat-killed BCG (c) were estimated by measuring IL-2 production in DE10 T-cell hybridoma culture supernatants ex vivo. The experiment was repeated at least three times
Analysis of MHC II, CIITA and T-cell costimulatory molecules on DCs following BCG infection
We measured the expression of cell surface markers involved in Ag presentation and T-cell interaction. Sorted inguinal LN DCs were stained with a panel of monoclonal antibodies (mAbs) to detect CD40, CD54, CD80, and CD86 by Flow Cytometry (FACS). No obvious regulation of CD40 or CD54 on DCs was observed during infection (Fig. 3a, b). High levels of CD80 and CD86 were noted at 12 h, but the presence of these markers had decreased by 72 h and 96 h (Fig. 3c, d). These results indicate that inguinal LN DCs undergo functional activation in the early stages of BCG infection.
Expression of MHC II, CIITA, and co-stimulatory molecules on DCs following BCG infection. Inguinal LNs were obtained at different time points following s.c. infection of mice with 1 × 108 CFU BCG (n = 6) and were sorted and stained with a panel of mAbs to detect cell-surface expression of CD40 (a), CD54 (b), CD80 (c), and CD86 (d) by FACS. Inguinal LNs were obtained from five groups of mice (n = 6) at different time points following s.c. injection of 1 × 108 CFU BCG. Transcription levels of MHC II, total CIITA (CIITA T), and CIITA type I (CIITA I) were analyzed using real-time PCR (e), and DCs were sorted and stained with mAbs to detect MHC II by FACS (f). The results are representative of three independent experiments and presented as means ± SEM. Statistical significance was determined using Student’s t-test (*P < 0.05)
We next investigated transcription levels of MHC II and CIITA transcription in inguinal LN DCs during BCG infection using real-time PCR. MHC II expression was found to be increased by BCG infection at a relatively slow rate, while total CIITA transcription was rapidly induced, and expression of CIITA type I declined between 48 and 96 h (Fig. 3e). In addition, to evaluate the expression of MHC II molecules involved in Ag presentation, sorted inguinal LN DCs were stained with mAbs for FACS analysis. All sorted DCs demonstrated up-regulation of MHC II molecules following the initiation of infection (Fig. 3f). The fact that the transcription and expression of MHC II proteins on the cell surface did not decline following BCG infection suggests that the expression and trafficking of MHC class II molecules may be not associated with the rapid loss of Ag85A peptide-MHC II complexes. As a result, it can be deduced that LN DCs do not provide a continuous source of mycobacterial Ag85A peptides for the formation of peptideMHC II complexes.
BCG infection kinetics of murine LN DCs
In order to monitor the presence of BCG bacilli in inguinal LN DCs following s.c. infection of mice and thus evaluate the infection rate of these immune cells, rBCG-GFP cells were used in combination with FACS. As might be expected, 0.4% of LN DCs exhibited green fluorescence after 12 h of infection, and this figure had increased to 2% by 96 h post-injection (Fig. 4a). We next determined whether BCG survives and multiplies within DCs over the course of infection. CFUs appeared at 4 h and had increased significantly by 12 h after s.c. administration of BCG to mice, and numbers remained elevated until the end of the experiment (Fig. 4b). These results suggest that BCG infected the inguinal LN DC 12 h post-infection and that it survives within the inguinal LN DC pool, representing a continuing source of mycobacterial Ag85A protein, with which LN DCs can form Ag85A peptide-MHCII complexes in vivo.
BCG infection kinetics of murine LN DCs. Six groups of mice (n = 6) were s.c. injected with 1 × 108 CFU rBCG-GFP, and LN cells were harvested at different time points. DCs were sorted and analyzed for the presence of rBCG-GFP. Infection of murine LN DCs with BCG (a). Six groups of mice (n = 6) were s.c. injected with 1 × 108 CFU BCG, and BCG in DCs was quantified in CFUs by culturing on Middlebrook 7H10 agar (b). The results are representative of three independent experiments and presented as means ± SEM. Statistical significance was determined using Student’s t-test (*P < 0.05, **P < 0.01, ***P < 0.001)
Discussion
CD4+ T-cell responses and the production of IFN-γ are particularly important to the containment of Mtb infection. In mice, between 1 and 3 weeks after initial infection, Mtb-specific T cells appear in the lungs, IFN-γ is expressed, and the bacterial burden is controlled [10]. Production of IFN-γ by splenocytes in response to Ag restimulation is observed within 6 days after i.v. Mtb infection [11]. To determine when the T-cell response is initiated, we obtained splenocytes and inguinal LN cells from mice 3, 6, and 9 days after s.c. BCG injection. Inguinal LN cells collected 6 days after infection produced IFN-γ in response to Ag85A restimulation. Thus, the T-cell immune response appears to have been initiated in the inguinal LN day 6 following BCG infection.
The mechanisms involved in MHC class II Ag processing and presentation, which are required for CD4+ T-cell activation, are crucial for controlling Mtb infection. Previous research investigated the kinetics of Ag-presenting activity by harvesting spleens following i.v. administration of rBCG expressing the E. coli MalE protein as a reporter Ag. The formation of MalE peptide-MHC complexes in splenic DCs was detected at 2, 4, and 12 h after rBCG infection, while MalE was barely detectable at 48 h [9]. However, this process has not yet been directly examined in LN DCs and by using an endogenous M. bovis BCG Ag. To investigate the dynamics of LN DCs Ag presentation, we harvested and sorted these cells from inguinal LNs at several time points after s.c. injection of mice with Ag85A protein or BCG and tested their capacity to stimulate DE10 T-cell hybridomas, which are specific for an immunodominant Ag85A peptide. In this manner, in vivo formation of Ag85A peptide-MHC complexes on DCs from BCG-injected mice was detected by measuring IL-2 production in DE10 T-cell hybridoma culture supernatants ex vivo. Ag85A peptide-MHC complexes on LN DCs appeared rapidly after inoculation, with IL-2 production being detected in response to DCs collected 4 h after BCG infection and the highest production in response to those harvested at 12 h. By contrast, IL-2 levels following exposure to DCs harvested 72 h after infection were barely detectable. Together, these results indicate that the MHC II presentation of mycobacteria-derived peptides by inguinal LN DCs is only transient, with Ag85A peptide-MHC II complexes on the surfaces of inguinal LN DCs disappearing rapidly. Some reports have shown that peptide-MHC complexes have a half-life of 25 h [12]. Thus, it can be concluded that the synthesis of Ag85A peptide-MHC II complexes on inguinal LN DCs was interfered.
Several reports have shown that Mtb and M. bovis inhibit intracellular processes associated with Ag presentation, including Ag processing, MHC class II expression, the trafficking of MHC class II molecules, and peptide-MHC class II binding [13, 14]. CIITA is the master transcriptional regulator of MHC class II molecules [15]. The transcription of CIITA itself is regulated by the three unique promoters pI, pIII, and pIV, which drive the expression of CIITA types I, III, and IV, respectively. pI is constitutively active in DCs [14]. In the current investigation, LN DCs exhibited the up-regulation of cell-surface MHC II molecules from 4 to 96 h following infection. Using real-time PCR, we analyzed the transcription levels of MHC II, total CIITA, and CIITA type I in DCs in response to BCG. Expression of MHC II was found to be induced by BCG infection relatively slowly, while total CIITA transcription was rapidly induced. This indicates that the transcription and expression of MHC II proteins on the cell surface did not declined following BCG infection, suggesting that the expression and trafficking of MHC class II molecules may be not associated with the rapid loss of Ag85A peptide-MHC II complexes. As a result, it can be deduced that LN DCs do not provide a continuing source of mycobacterial Ag85A peptides for the formation of peptide-MHC II complexes.
Considerable evidence shows that DCs can phagocytose mycobacteria and may be the first cells to encounter such pathogens, therefore, DCs are likely to be responsible for initiating the subsequent immune response. The survival of mycobacteria within DCs has been assessed previously in vitro using the BCG vaccine strain and virulent M. bovis, both of which were shown to be phagocytosed by DCs after 24 h of infection [16]. Mtb cells disseminate to draining LNs within 8 days following respiratory infection [17]. Approximately 2% of the splenic DC population (CD11c+ cells) was found to contain BCG at 4 h following i.v. infection [9]. In the present study, the presence of rBCG-GFP bacilli in inguinal LN DCs following s.c. inoculation of mice was monitored by FACS. As expected, the percentage of infected DCs increased to 2% after 96 h of infection. We then examined whether mycobacteria survive and multiply within DCs during infection. Following s.c. administration of BCG to mice, CFUs appeared at 4 h, increased significantly by 12 h, remaining elevated until the last time point. These results suggest that BCG survives within the inguinal LN DC pool, representing a continuing source of mycobacterial Ag85A protein with which LN DCs can form Ag85A peptide-MHCII complexes in vivo. Some reports have shown that live Mtb can alter phagosome maturation and decrease Ag processing, providing a mechanism for Mtb to evade immune surveillance and enhance its survival within the host [18,19,20]. Based on our findings, we conclude that the inhibition of Ag processing due to the reduced production of Ag85A peptide is the primary reason for the rapid loss of Ag85A peptide-MHC II complexes.
Conclusions
In the present study, we evaluated the in vivo dynamics of early Ag presentation by murine LN DCs in response to M. bovis BCG Ag85A protein. Our results showed that the early Ag-presenting activity of murine DCs induced by M. bovis BCG Ag85A protein in vivo was transient and that the inhibition of Ag processing induced by a decrease in the production of Ag85A peptide is the primary reason for the rapid loss of Ag85A peptide-MHC II complexes and the short duration of Ag presentation by LN DCs during BCG infection in vivo.
Methods
Experimental animals
Six-week-old female C57BL/6 mice were purchased from Vital River (Beijing, China). The mice were housed, handled, and immunized at our animal biosafety facilities, and all procedures were approved by the Institutional Animal Experimental Committee of Yangzhou University. All experiments were performed according to the national guidelines for animal welfare. The mice were euthanized by cervical dislocation under isoflurane, and spleens and inguinal LNs were collected for analysis.
Bacterial strains and culture conditions
M. bovis BCG Pasteur 1173P2 and rBCG expressing GFP (rBCG-GFP) were kindly provided by Dr. Xiaoming Zhang (Institut Pasteur of Shanghai, Chinese Academy of Sciences, Shanghai, China). Both strains were grown with gentle agitation (80 rpm) in Middlebrook 7H9 medium (Difco, Detroit, MI, USA) supplemented with 0.05% Tween 80 and 10% albumin-dextrose-catalase (ADC) enrichment or on solid Middlebrook 7H10 medium (Difco) supplemented with 0.05% Tween 80 and 10% oleic-ADC enrichment.
T-cell hybridoma and Ags
MHC II-restricted DE10 T-cell hybridomas specific for the Mtb Ag85A peptide comprising amino acids 241 to 260 [21] were kindly provided by Dr. Claude Leclerc (Institut Pasteur, Paris, France). The Ag85A protein was constructed and expressed in our laboratory, and the Ag85A peptide (amino acids 241–260) was synthesized by SciLight Biotechnology (Beijing, China).
Detection of IFN-γ production following BCG infection
C57BL/6 mice were s.c. vaccinated with 1 × 108 CFU BCG and sacrificed 3, 6, and 9 days later, at which point, spleens and inguinal LNs were removed aseptically and transferred to complete RPMI-1640 medium for preparation of single-cell suspensions. The mononuclear cells, isolated using Histopaque 1083 (Sigma, St. Louis, MO, USA), were seeded at 1 × 106 cells/well in 96-well plates containing complete RPMI-1640 medium. They were subsequently stimulated with 10 μg/ml Ag85A peptide, 10 μg/ml Ag85A protein, or 5 μg/ml bovine PPD (Prionics, Schlieren, Switzerland) and incubated at 37 °C in an atmosphere of 5% CO2 in air. Supernatants were then harvested at 48 h post-stimulation, frozen, and later tested for IFN-γ concentration by sandwich enzyme-linked immunosorbent assay (ELISA, BD Biosciences, Franklin Lakes, NJ, USA).
Ag presentation assay
C57BL/6 mice were s.c. injected with 1 × 108 CFU BCG or heat-killed BCG in 200 μl PBS or with PBS alone. Mice were sacrificed at various time points, and their inguinal LNs removed and perfused with 400 U/ml collagenase type IV (Invitrogen, Carlsbad, CA, USA) containing 50 μg/ml DNase I (Invitrogen). Single LN-cell suspensions were prepared, and DCs were sorted with an autoMACS separator (Miltenyi Biotec, Bergisch Gladbach, Germany) using CD11c as a cell marker. Specifically, LN cells were first incubated with anti-CD11c MicroBeads (Miltenyi Biotec) before autoMACS separation, resulting in a population of CD11chigh cells (DCs). The purity of these murine LN DCs was then analyzed using a FACSCalibur instrument (BD Biosciences). For the ex vivo Ag presentation assay itself, the purified LN DCs were transferred to 96-well microplates and serially diluted in complete RPMI-1640 medium. DE10 T-cell hybridomas at a density of 1 × 105/well were then added, and after incubation for 24 h, supernatants were collected, frozen, and later tested for IL-2 content by sandwich ELISA (BD Biosciences).
Cell phenotype analysis
C57BL/6 mice were s.c. injected with 1 × 108 CFU BCG in 200 μl PBS or with PBS alone. The mice were sacrificed after various periods for the preparation of single inguinal LN-cell suspensions and sorting of DCs by autoMACS. FITC-conjugated anti-CD11c, and biotinylated anti-I-Ad, anti-CD40, anti-CD54, anti-CD80, and anti-CD86 antibodies were used to label cells. Allophycocyanin-conjugated streptavidin was employed to visualize biotin conjugates. A FACSCalibur and FlowJo software (FlowJo LLC, Ashland, OR, USA) were then used for multicolor staining analysis of the labeled cells. DCs were sorted using the autoMACS system before being pelleted and resuspended in lysis buffer. Cellular RNA was purified with an RNeasy kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions, and total RNA was reverse-transcribed into cDNA using SuperScript reverse transcriptase (Thermo Fisher Scientific, Waltham, MA, USA).
In vivo infection assay
C57BL/6 mice were s.c. injected with 1 × 108 CFU BCG or rBCG-GFP in 200 μl PBS or with PBS alone. Mice were sacrificed at various time points, and single inguinal LNs were removed aseptically and transferred to complete RPMI-1640 medium. Single-cell suspensions were prepared, and DCs were sorted with an autoMACS separator as above. The percentage of DCs infected with rBCG-GFP was analyzed using a FACSCalibur instrument and FlowJo software. BCG-infected DCs were pelleted and resuspended in lysis buffer. Ten-fold serial dilutions of these suspensions were then plated on solid Middlebrook 7H10 medium, and colonies were counted after incubation at 37 °C for 2–3 weeks.
Statistical analysis
All data are expressed as means ± SE. Statistical analysis was performed by Student’s t-test using GraphPad Prism software. P values < 0.05 were considered statistically significant.
Abbreviations
- ADC:
-
Albumin-dextrose-catalase
- APC:
-
Antigen-presenting cell
- BCG:
-
Bacillus Calmette–Guérin
- CIITA:
-
MHC class II transactivator
- DC:
-
Dendritic cell
- LN:
-
Lymph node
- MHC II:
-
Major histocompatibility complex class II
- Mtb :
-
Mycobacterium tuberculosis
- PPD:
-
Purified protein derivative
- rBCG:
-
Recombinant BCG
- TB:
-
Tuberculosis
References
Aguilo N, Gonzalo-Asensio J, Alvarez-Arguedas S, Marinova D, AB Gomez S, Uranga R, Spallek M, Singh M, Audran R, Spertini F, et al. Reactogenicity to major tuberculosis antigens absent in BCG is linked to improved protection against Mycobacterium tuberculosis. Nat Commun. 2017;8:16085.
Kaveh DA, Bachy VS, Hewinson RG, Hogarth PJ. Systemic BCG immunization induces persistent lung mucosal multifunctional CD4 T(EM) cells which expand following virulent mycobacterial challenge. PLoS One. 2011;6:e21566.
Vogelzang A, Perdomo C, Zedler U, Kuhlmann S, Hurwitz R, Gengenbacher M, Kaufmann SH. Central memory CD4+ T cells are responsible for the recombinant Bacillus Calmette-Guerin DeltaureC::hly vaccine's superior protection against tuberculosis. J Infect Dis. 2014;210:1928–37.
Kaufmann E, Spohr C, Battenfeld S, De Paepe D, Holzhauser T, Balks E, Homolka S, Reiling N, Gilleron M, Bastian M. BCG vaccination induces robust CD4+ T cell responses to Mycobacterium tuberculosis complex-specific Lipopeptides in Guinea pigs. J Immunol. 2016;196:2723–32.
Lindestam Arlehamn CS, McKinney DM, Carpenter C, Paul S, Rozot V, Makgotlho E, Gregg Y, van Rooyen M, Ernst JD, Hatherill M, et al. A quantitative analysis of complexity of human pathogen-specific CD4 T cell responses in healthy M. Tuberculosis infected south Africans. PLoS Pathog. 2016;12:e1005760.
Griffiths KL, Ahmed M, Das S, Gopal R, Horne W, Connell TD, Moynihan KD, Kolls JK, Irvine DJ, Artyomov MN, et al. Targeting dendritic cells to accelerate T-cell activation overcomes a bottleneck in tuberculosis vaccine efficacy. Nat Commun. 2016;7:13894.
Lozza L, Farinacci M, Bechtle M, Staber M, Zedler U, Baiocchini A, Del Nonno F, Kaufmann SH. Communication between human dendritic cell subsets in tuberculosis: requirements for naive CD4(+) T cell stimulation. Front Immunol. 2014;5:324.
Torres M, Ramachandra L, Rojas RE, Bobadilla K, Thomas J, Canaday DH, Harding CV, Boom WH. Role of phagosomes and major histocompatibility complex class II (MHC-II) compartment in MHC-II antigen processing of Mycobacterium tuberculosis in human macrophages. Infect Immun. 2006;74:1621–30.
Jiao X, Lo-Man R, Guermonprez P, Fiette L, Deriaud E, Burgaud S, Gicquel B, Winter N, Leclerc C. Dendritic cells are host cells for mycobacteria in vivo that trigger innate and acquired immunity. J Immunol. 2002;168:1294–301.
Harding CV, Boom WH. Regulation of antigen presentation by Mycobacterium tuberculosis: a role for toll-like receptors. Nat Rev Microbiol. 2010;8:296–307.
Tian T, Woodworth J, Skold M, Behar SM. In vivo depletion of CD11c+ cells delays the CD4+ T cell response to Mycobacterium tuberculosis and exacerbates the outcome of infection. J Immunol. 2005;175:3268–72.
Lanzavecchia A, Reid PA, Watts C. Irreversible association of peptides with class II MHC molecules in living cells. Nature. 1992;357:249–52.
Chang ST, Linderman JJ, Kirschner DE. Multiple mechanisms allow Mycobacterium tuberculosis to continuously inhibit MHC class II-mediated antigen presentation by macrophages. Proc Natl Acad Sci U S A. 2005;102:4530–5.
Srivastava S, Grace PS, Ernst JD. Antigen export reduces antigen presentation and limits T cell control of M. Tuberculosis. Cell Host Microbe. 2016;19:44–54.
Ghorpade DS, Holla S, Sinha AY, Alagesan SK, Balaji KN. Nitric oxide and KLF4 protein epigenetically modify class II transactivator to repress major histocompatibility complex II expression during Mycobacterium bovis bacillus Calmette-Guerin infection. J Biol Chem. 2013;288:20592–606.
Hope JC, Thom ML, McCormick PA, Howard CJ. Interaction of antigen presenting cells with mycobacteria. Vet Immunol Immunopathol. 2004;100:187–95.
Chackerian AA, Alt JM, Perera TV, Dascher CC, Behar SM. Dissemination of Mycobacterium tuberculosis is influenced by host factors and precedes the initiation of T-cell immunity. Infect Immun. 2002;70:4501–9.
Ramachandra L, Noss E, Boom WH, Harding CV. Processing of Mycobacterium tuberculosis antigen 85B involves intraphagosomal formation of peptide-major histocompatibility complex II complexes and is inhibited by live bacilli that decrease phagosome maturation. J Exp Med. 2001;194:1421–32.
Sendide K, Deghmane AE, Pechkovsky D, Av-Gay Y, Talal A, Hmama Z. Mycobacterium bovis BCG attenuates surface expression of mature class II molecules through IL-10-dependent inhibition of cathepsin S. J Immunol. 2005;175:5324–32.
Singh CR, Moulton RA, Armitige LY, Bidani A, Snuggs M, Dhandayuthapani S, Hunter RL, Jagannath C. Processing and presentation of a mycobacterial antigen 85B epitope by murine macrophages is dependent on the phagosomal acquisition of vacuolar proton ATPase and in situ activation of cathepsin D. J Immunol. 2006;177:3250–9.
Johansen P, Fettelschoss A, Amstutz B, Selchow P, Waeckerle-Men Y, Keller P, Deretic V, Held L, Kundig TM, Bottger EC, et al. Relief from Zmp1-mediated arrest of phagosome maturation is associated with facilitated presentation and enhanced immunogenicity of mycobacterial antigens. Clin Vaccine Immunol. 2011;18:907–13.
Acknowledgements
The authors are grateful to Dr. Claude Leclerc (Institut Pasteur, Paris, France) and Dr. Xiaoming Zhang (Institut Pasteur of Shanghai, Chinese Academy of Sciences, Shanghai, China).
Funding
This work was supported in part by the National Key Research and Development Program of China (2017YFD0500300), the National Natural Science Foundation of China (31602031), the Science and Technology Program of Jiangsu (BK20160466, 16KJB230003, BK20171285 and BK20170493), and the Qinglan Project, Six Talent Peaks Project and Priority Academic Development Program of Jiangsu Higher Education Institutions (PADP).
Availability of data and materials
All data generated or analyzed during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Contributions
ZZ X, XC and XA J designed the experiments. ZZ X, AH X, XL, ZC Z, YC S, SS J, TL, YQ X, HW, and CM performed the experiments and analyzed the data. LS, YL Y, XC, and XA J contributed reagents/materials/analysis tools. ZZ X, XC, and XA J wrote and revised the paper. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
The mice were housed, handled, and immunized at our animal biosafety facilities, and all procedures were approved by the Institutional Animal Experimental Committee of Yangzhou University. All experiments were performed according to the national guidelines for animal welfare.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Xu, Z., Xia, A., Li, X. et al. Rapid loss of early antigen-presenting activity of lymph node dendritic cells against Ag85A protein following Mycobacterium bovis BCG infection. BMC Immunol 19, 19 (2018). https://doi.org/10.1186/s12865-018-0258-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12865-018-0258-8