Abstract
Background
With declining wild fish populations, farmed salmon has gained popularity as a source for healthy long-chain highly unsaturated fatty acids (LC-HUFA). However, the introduction of plant oil in farmed salmon feeds has reduced the content of these beneficial LC-HUFA. The synthetic capability for LC-HUFAs depends upon the dietary precursor fatty acids and the genetic potential, thus there is a need for in-depth understanding of LC-HUFA synthetic genes and their interactions with other genes involved in lipid metabolism. Several key genes of LC-HUFA synthesis in salmon belong to the fatty acid desaturases 2 (fads2) family. The present study applied whole transcriptome analysis on two CRISPR-mutated salmon strains (crispants), 1) Δ6abc/5Mt with mutations in Δ5fads2, Δ6fads2-a, Δ6fads2-b and Δ6fads2-c genes, and 2) Δ6bcMt with mutations in Δ6fads2-b and Δ6fads2-c genes. Our purpose is to evaluate the genetic effect fads2 mutations have on other lipid metabolism pathways in fish, as well as to investigate mosaicism in a commercial species with a very long embryonal period.
Results
Both Δ6abc/5Mt and Δ6bcMt crispants demonstrated high percentage of indels within all intended target genes, though different indel types and percentage were observed between individuals. The Δ6abc/5Mt fish displayed several disruptive indels which resulted in over 100 differentially expressed genes (DEGs) enriched in lipid metabolism pathways in liver. This includes up-regulation of srebp1 genes which are known key transcription regulators of lipid metabolism as well as a number of down-stream genes involved in fatty acid de-novo synthesis, fatty acid β-oxidation and lipogenesis. Both elovl5 and elovl2 genes were not changed, suggesting that the genes were not targeted by Srebp1. The mutation of Δ6bcMt surprisingly resulted in over 3000 DEGs which were enriched in factors encoding genes involved in mRNA regulation and stability.
Conclusions
CRISPR-Cas9 can efficiently mutate multiple fads2 genes simultaneously in salmon. The results of the present study have provided new information on the transcriptional regulations of lipid metabolism genes after reduction of LC-HUFA synthesis pathways in salmon.
Similar content being viewed by others
Background
Atlantic salmon (Salmo salar L.) is a popular fish species for human consumption since it contains high amounts of long-chain highly unsaturated fatty acids (LC-HUFA) such as docosahexaenoic acid (22:6n-3, DHA), eicosapentaenoic acid (20:5n-3, EPA) and arachidonic acid (20:4n-6, ARA). The high LC-HUFA content in farmed salmon originates mainly from dietary inclusions of marine fish oil and fish meal. However, traditional marine fisheries have been exploited to their limits, and with increasing volume of salmon production, dietary marine oil and meal sources have been gradually diluted over the past decades. Plant oils are used to substitute marine oils in aquaculture diets, with an increasing levels from 0% of total lipids in 1990 to 19.2% in 2013 [1]. This has resulted in a reduction of LC-PUFA levels in salmon flesh since plant oils do not contain LC-PUFA [2].
Salmon are capable of synthesizing LC-HUFA through elongation and desaturation of α-linolenic (18:3n-3) and linoleic (18:2n-6) acids, and the synthesis is often increased when the fish are given a plant oil diet with low LC-HUFA [3]. This explains the fact that salmon can tolerate partial substitution of fish oil with plant oil without negative impact on growth rate, feed conversion or any histopathological lesions [4]. However, the synthesized LC-HUFA in salmon is still not enough to compensate for the reduced LC-HUFA level caused by inclusion of plant oil in diet [2]. Thus, salmon has limited capability in bioconverting the precursors, 18:3n-3 and 18:2n-6 to essential LC-HUFAs [5, 6]. In order to further improve the LC-HUFA synthetic capacity in salmon, a better understanding of the regulation of genes involved in LC-HUFA synthesis is needed.
The pathways of LC-HUFA synthesis in salmon involves 4 elongases encoded by elovl2, elovl4, elovl5a and elvol5b and 4 desaturases encoded by Δ5fads2, Δ6fads2-a, Δ6fads2-b and Δ6fads2-c. All 8 genes have been cloned and functionally characterised through heterologous expression in yeast (Saccharomyces cerevisiae) [7, 8]. Both elovl5a and elovl5b are mainly involved in elongating C18 and C20 fatty acids, while elovl2 and elovl4 are involved in elongating C20 and C22 [8,9,10]. All four fads genes in salmon are homologs to the human FADS2 gene. In salmon they have separate functions where double bonds are introduced at C5 (Δ5fads2) or C6 (Δ6fads2-a, Δ6fads2-b and Δ6fads2-c) from the carboxyl end [10, 11]. Feeding of plant oil often leads to up-regulation of both elovl and fads2 genes in salmon, which is likely due to the low LC-HUFA content in the diet [5, 12,13,14].
In addition to the LC-HUFA synthesis genes, many other genes involved in fatty acid de-novo synthesis, fatty acid oxidation and cholesterol biosynthesis are also differentially expressed after feeding plant oil [5, 12,13,14]. It is difficult to conclude the reason for the differential expression of lipid metabolism genes since plant oils are devoid of cholesterol and LC-HUFA, and contain high amounts of C18 PUFA precursors and phytosterols compared to fish oil [15,16,17]. In a recent study, we disrupted the LC-HUFA synthesis pathway in salmon by mutating elovl2 gene using CRISPR/Cas9 technology [18]. In addition to a decreased DHA content in mutant fish, we were able to identified up-regulation of fads2 genes as well as several genes involved in fatty acid biosynthesis and lipogenesis as consequence of the knock out [18]. This suggests a systemic change of lipid metabolism regulation in response to the disruption of LC-HUFA synthesis in salmon.
CRISPR/Cas9 technology has recently been used in salmon to edit genes and generate mutants for elovl2, slc45a2 and dnd [18,19,20,21]. Both guide RNA (gRNA) and Cas9 mRNA are injected into one-cell stage salmon embryos to induce a targeted double-strand break, followed by non-homologous end joining (NHEJ) which generates random insertions and deletions (indels) at the target sites that can lead to a non-functional protein. However, because of a three-year generation interval, the generation of homozygous edited salmon is too tedious for research projects. Genetic manipulation efficacy in the founder generation largely depend upon target gene and gRNA design, but there is also a need to address how mosaics differ in the tissues and affects function of the encoded gene product. For this species it is therefore necessary to optimize editing efficiency and reduce the problem of mosaicism in the F0 generation. Compared to teleost model species, the Atlantic salmon embryo develops slowly and hatches after about 80 days, or 500-day degrees (days x temperature in oC). This developmental pace may lead to degradation of CRISPR components such as CAS9 mRNA or protein and guide RNA’s which may have an impact upon mosaicism.
We have recently used CRISPR/Cas9 to mutate fads2 genes in salmon which resulted in down-regulation of targeted genes and lower DHA and EPA contents in tissues [22]. However, the impact of impaired LC-HUFA biosynthesis on the regulation of other genes - both from lipid metabolism and globally - was still unclear. In the present study we aimed to further characterize transcriptional regulation of lipid metabolism in fads2-mutated salmon by comparing their transcriptomes to wildtype fish. Our study also seeks to provide detailed insights on the effect and distribution of genetic mosaicism in salmon individuals after mutation of fads2 genes.
Result and discussion
CRISPR/Cas9 induced mutations
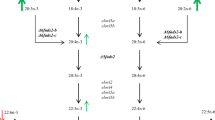
The two strains of Atlantic salmon carrying CRISPR/Cas9-mediated mutations were generated as described earlier [22]. In both strains CRISPR/Cas9 mediated mutations were induced using a single CRISPR gRNA targeting multiple genes (Fig. 1a). The gRNA of Δ6abc/5Mt salmon targeted Δ6fads2-a, Δ6fads2-b, Δ6fads2-c and Δ5fads2 genes, while the gRNA of Δ6bcMt targeted Δ6fad2s-b and Δ6fads2-c. Both Δ6abc/5 and Δ6bc mutant salmon were co-injected with a CRISPR gRNA targeting slc45a2 which induces an albino phenotype and served as visual control in our experiment.
a Circos plot showing the different target sites of the CRISPR gRNAs. Gene ∆5fads2, ∆6fads2-a and ∆6fads2-c have multiple transcripts while yellow boxes indicate exons of each transcript. b: Boxplot showing the maximum proportion of insertions/deletions (indels) within the CRISPR gRNA target site as identified by AmpliSeq. Different color indicates liver (L) or white muscle (WM) tissues from WT, ∆6abc/5 mutant or ∆6bc mutant salmon. Each dot indicates L or WM tissue of an individual fish. c: Bar plots showing the (SnpEff) predicted impact of the indel on the respective main transcript by individual. Impacts are classified as: HIGH = The variant is assumed to have high (disruptive) impact in the protein; MODERATE = A non-disruptive variant that might change protein effectiveness; LOW = The variant is assumed to be mostly harmless; WT = Wild type/no indel. Each bar of the figure represents data of an individual fish
CRISPR/Cas9-induced structural mutations at the fads2 as well as the slc45a2 genes of fish from both Δ6abc/5Mt and Δ6bcMt strains were confirmed by using AmpliSeq. All fish injected with CRISPR/Cas9 carried structural variants at the respective gRNA target sites (Fig. 1 b). For all individuals from both CRISPR strains we observed a high degree of mosaicism at each of the respective gRNA target sites (Fig. 1b). This suggests that Cas9-induced editing continues after the one-cell stage of the embryos. In order to better understand the consequences of the different structural variants on a phenotypic level, we predicted variant effects using SnpEff and summarised the results according to the impact category (Fig. 1c). The majority of structural variants across all individuals were predicted to have “high” impact, meaning to have a likely disruptive effect on the protein function. Nevertheless, our analysis also showed that many of the individuals from the two CRISPR strains still carried a considerable amount of the WT genotype (non-CRISPR mutated). Therefore, we believe it is more correct to consider the two resulting CRISPR strains as fads2 knock-downs rather than knockouts. The Δ6abc/5Mt gRNA targeted sequence right after the cytochrome b5-like domain of fads2 genes, while Δ6bcMt gRNA targeted sequences on exon 1 before all protein domains. Therefore, the out-of-frame mutations in Δ6abc/5Mt and Δ6bcMt were expected to disrupt characteristic domains identified in fatty acyl desaturases, though our CRISPR-target sites did not specifically fall within protein domains. These out-of-frame mutations identified by Ampliseq could explain the nonsense-mediated decay (NMD) of the mutant mRNA and impaired biosynthesis of LC-PUFA in Δ6abc/5Mt fish [22].
CRISPR/Cas9-induced indels cause Δ6fads2-a exon skipping events
Interestingly, we found that CRISPR/Cas9 induced mutations of Δ6abc/5Mt gRNA in the Δ6fads2-a gene were affecting splicing of exonic part 6 (harbouring the CRISPR target site; exonic part 6 corresponds to exon 4 in transcript: XM_014170212.1; exon 3 in XM_014170213.1). Analysis of exonic-part 6 retention in Δ6abc/5-mutated salmon using RNA-seq data revealed mis-splicing of the Δ6fads2-a transcript resulting in the skipping of exonic part 6 (Fig. 2). Exon skipping caused by CRISPR/Cas9-generated mutations was observed previously in both cell lines [23, 24] and genetically modified organisms including zebrafish [25] and salmon [18]. CRISPR induced mis-splicing is mostly caused by one of two mechanisms: i) indels generated by a CRISPR-mutation affects the exon-intron boundaries or ii) indels promote exon skipping by disrupting an exon splicing enhancer or introducing an exon splicing silencer within the targeted exon [26]. However, neither mechanism fits to our study. This was because other Δ6abc/5Mt gRNA target sites on Δ5fads2, Δ6fads2-b and Δ6fads2-c genes contained identical sequences and showed the same distance to exon-intron boundaries, but did not affect splicing. Nonetheless, the skipping of exon 6 in Δ6fads2-a transcripts will result in the production of truncated proteins that lack 37 amino acids, which suggests deleterious effects on protein structure and functions.
Detection of exon skipping in Δ6fads2-a in relation to CRISPR. a: Exon structure for the three transcripts encoded by Δ6fads2-a. The targeting site (s1) for the Δ6abc/5Mt gRNA is enlarged and highlighted in red. b: Schematic drawing on how aligned RNA-seq reads were used to calculate the percentage of exon retention (PER) for a sample. c: Exon skipping was confirmed by using the aligned RNA-seq reads to calculate the PER for each sample (represented as point)
CRISPR-targeted fads2 genes are down-regulated in the liver of Δ6abc/5 but not Δ6bc salmon
Many of the CRISPR induced structural variants introduce premature termination codons likely to trigger mRNA degradation by nonsense-mediated decay (NMD) [27]. Indeed, we found that CRISPR-targeted Δ5fads2, Δ6fads2-a and Δ6fads2-b genes were strongly down-regulated (q < 0.05) in Δ6abc/5Mt salmon compared to WT regardless of the dietary treatment (Fig. 3). In Δ6bcMt salmon, the CRISPR-targeted Δ6fads2-b gene was down-regulated compared to WT, but the levels of down-regulation were less clear than in Δ6abc/5Mt salmon. Surprisingly, the expression of Δ5fads2 and Δ6fads2-a genes was also down-regulated in Δ6bcMt salmon, though both genes were not targeted by Δ6bcMt gRNAs. The expression of Δ6fads2-c gene was generally very low, suggesting that it is unlikely to play a major role in salmon liver. This low level expression may also explain that Δ6fads2-c was not affected by CRISPR mutations (Fig. 3). The expression of other genes in the LC-HUFA synthesis pathway, elovl2, elovl5-a and elovl5-b, was stable between Δ6abc/5Mt, Δ6bcMt and WT salmon.
Expression of LC-HUFA synthesis genes in wildtype (WT), Δ6abc/5Mt and Δ6bcMt salmon fed with either plant oil or fish oil diet. Gene expression are shown in transcript per million (TPM) value which is raw counts normalised by both library size and mRNA length. Different letter indicates genes which were differentially expressed (q < 0.05 & |log2FC| > 0.5)
The NMD-mediated mRNA degradation, absence of exon 6 in Δ6fads2-a transcripts, and other CRISPR-induced mutations such as out-of-frame mutations are expected to produce non-functional enzyme proteins that would ultimately disrupt LC-HUFA biosynthesis in the fish. Indeed, analysis of tissue composition of LC-HUFA coupled with assays of desaturation and elongation activities in liver showed clear impacts of the CRISPR-mutations. The mutation of Δ6abc/5 genes in salmon resulted in significant reduction of DHA and EPA in phospholipids compared to WT [22]. On the other hand, we observed effects of background wildtype alleles in the Δ6abc/5Mt salmon (Fig. 1b and c) accounting for limited but measurable desaturation activities [22].
Transcriptional changes in liver after mutating fads2 genes
An average of 29 million reads were mapped on to the salmon genome ICSASG_v2. From a total of 55,304 annotated genes, 23,114 genes had at least 1 count per million (CPM) in 25% of the samples, and were considered for subsequent analysis. By applying principal component analysis (PCA) on Log2 CPM of the top 1000 most variant genes, we identified a clear separation of plant oil and fish oil samples between PC1 (explaining 34.8% of the observed variation) and PC2 (8.3%) as well as a separation of WT and Δ6abc/5Mt samples between PC2 and PC3 (6.8%) (Fig. 4). Although not as strong, we also found a clear tendency for separation of WT and Δ6bcMt samples between PC2 and PC3. Plant oil diets and CRISPR-mutation seemed to have different impacts on gene transcription in salmon liver, though both the diet and mutation have generated low levels of LC-HUFA in the fish body. The 20 most variant genes are listed in Supplementary Table 3.
Principle component analysis (PCA) on Log2 count per million (CPM) of the top 1000 most variant genes between all liver samples. Different colors represents genetic groups of WT, Δ6abc/5-mutated and Δ6bc-mutated salmon, while the color intensity represents different dietary treatments of either plant oil (low HUFA) diet or fish oil diet (high HUFA)
Differential expression analysis (DEA) was done by contrasting crispants and WT salmon separately under plant oil and fish oil diets. This resulted in 121 differentially expressed genes (DEGs, q < 0.05 & |log2FC| > 0.5) in Δ6abc/5Mt salmon compared to WT when fed a fish oil diet, while 104 DEGs were found between crispant and WT salmon under a plant oil diet (Fig. 5 a). Surprisingly, more DEGs were found in Δ6bcMt salmon compared to WT. This includes 1156 genes identified in crispant salmon when fed a fish oil diet and 1348 DEGs identified in salmon fed a plant oil diet. A total number of 3987 DEGs was found in WT salmon fed a plant oil diet compared to fish oil, while the numbers of diet-associated DEGs were 4179 and 2057 in Δ6abc/5Mt and Δ6bcMt fish respectively.
Differential expression analysis in liver between wildtype (WT) and mutated salmon. a Number of up-regulated and down-regulated differential expressed genes (DEGs, q < 0.05 & |log2FC| > 0.5) either between WT and Δ6abc/5-mutated salmon, or between WT and Δ6bc-mutated salmon, or between WT salmon fed plant oil and fish oil. b Significantly (p < 0.005) enriched KEGG pathways of the DEGs. Hypergeometric test was applied based on the number of DEGs versus total genes annotated to each KEGG pathway
To further understand the functions of DEGs between crispant and WT salmon, we conducted a KEGG enrichment analysis by comparing the number of DEGs to the total number of genes in each KEGG pathway (Fig. 5 b). The DEGs of Δ6abc/5Mt salmon were not only enriched in the fatty acid metabolism pathway, but also the peroxisome proliferator-activated receptors (PPAR) signalling pathway which is involved in many metabolic pathways including fatty acid synthesis and catabolism [28]. This supports previous studies, indicating PPAR to be the key transcriptional regulator of fatty acid metabolism in salmon [3]. Differential regulation of these pathways was likely caused by decreased EPA and DHA, and consequential accumulation of 18:3n-3 and 18:2n-6 after disruption of the LC-HUFA synthesis pathway [22]. Accumulated 18:3n-3 and 18:2n-6 could not be synthesised further to DHA and EPA after disruption of fads2 genes. Instead they were most likely consumed by β-oxidation which was activated by the PPAR transcription factor [28]. Similar enrichment of fatty acid metabolism and PPAR signalling pathways was also found in the DEGs between WT salmon fed plant oil and fish oil (Fig. 5 b). Additionally, the sterol biosynthesis pathway was enriched for DEGs between WT salmon fed plant oil and fish oil, but was not enriched for the DEGs between fads2 mutants versus WT fish (Fig. 5 b). Indicating that the LC-HUFA level and PPAR has little effect on cholesterol biosynthesis in salmon, which is more likely regulated by other biochemical signals such as low cholesterol level and other transcription factors including sterol regulatory binding protein 2 (SREBP2) [12, 13, 15]. Many other pathways were also enriched for the DEGs of WT fed plant oil versus fish oil, such as amino acid biosynthesis and RNA transport. This suggests that dietary inclusion of plant oil has more complex impact on salmon than just reducing LC-HUFA and cholesterol levels in the fish body. Our study has successfully separated the effect of low LC-HUFA level from other effects of plant oil inclusion, however more research is required to understand the complete regulatory network in response to the change of plant oil in the diet. Surprisingly, no lipid metabolism pathways were enriched in Δ6bcMt salmon compared to WT, regardless of dietary LC-HUFA level. This was in accordance to the fatty acid composition in liver, where no significant difference was found between Δ6bcMt salmon and WT [22]. The DEGs were likely more enriched in mRNA regulation pathways, including mRNA surveillance and spliceosome pathways. Nevertheless, the reason for the high number of DEGs in Δ6bcMt salmon and their enriched pathways needs to be further investigated.
Expression of lipid metabolism genes in response to Δ6abc/5 mutation
Due to many unexpected and lipid metabolism unrelated DEGs found in Δ6bcMt salmon, only Δ6abc/5Mt fish were included for further transcriptomic analysis to understand the transcriptional regulation of lipid metabolism after disrupting LC-HUFA synthesis genes. Here we discussed DEGs of lipid metabolism pathways that were enriched in Δ6abc/5Mt versus WT salmon, aiming to understand the regulatory network of lipid metabolism genes in response to Δ6abc/5Mt. The Δ6abc/5 mutant showed 14 (13.4%) differentially expressed lipid metabolism genes when fed plant oil diet, while fewer (7 genes, 5.8%) lipid DEGs were identified in salmon fed the fish oil diet (Supplementary Table 1). The higher numbers of DEGs in Δ6abc/5Mt salmon fed the plant oil diet suggest a compensatory response to the combined effects of impaired endogenous LC-HUFA biosynthesis and reduced dietary LC-HUFA levels. On the other hand, the reduced number of lipid DEGs in Δ6abc/5Mt salmon fed the fish oil diet suggests an impact of dietary LC-HUFA levels on gene transcription, most likely an end-product-mediated inhibition. Nevertheless, 4 lipid DEGs were identified in Δ6abc/5Mt fish fed both plant oil and fish oil experimental diets including Δ5fad, Δ6fad-a, abcd1 and acc2. Besides the two CRISPR-targeted genes, the down-regulation of acc2 and up-regulation of abcd1 suggests an increase of the fatty acid β-oxidation pathway for energy expenditure after CRISPR-mutation [29].
Low levels of LC-HUFA often induce hepatic expression of Δ5fads2 and Δ6fads2-a genes as shown in our previous elovl2-mutated salmon [18]. On the other hand, reduced DHA level has little effect on the expression of elovl5 and elovl2 genes as shown in the present Δ6abc/5Mt salmon (Fig. 3). However, the expression of elovl2 and elovl5 genes are often up-regulated in fish fed plant oil compared to fish oil diets (Fig. 3) [30, 31]. Although plant oil diets also contains lower DHA and EPA, our data has shown that the expression of elovl genes was more likely induced by other differences between fish oil and plant oil diets. Sterol regulatory element binding proteins (SREBPs) are suggested to be involved in regulating lipid metabolism in both mammals and fish [32, 33]. Atlantic salmon has four srebp1 paralogous genes, srebp1a, srebp1b, srebp1c and srebp1d which are all orthologs of the zebrafish srebp1 gene (Supplementary Table 1). Both Δ6abc/5Mt and low LC-HUFA diets resulted in increased transcription of all four srebp1 genes in salmon (Fig. 6 and Supplementary Table 1). The transcription of the srebp1 genes was negatively (p < 0.05) correlated to the DHA level in phospholipids. On the other hand, transcription of srebp2 genes were not up-regulated in mutated versus WT salmon, and are not correlated to DHA level (Fig. 6 b). The different regulation of srebp1 and srebp2 transcription is consistent with previous studies in mammals, suggesting that srebp1 transcription is regulated by DHA levels in salmon, while srebp2 transcription is more likely to be induced by low cholesterol levels in the plant oil diet [32].
Expression change of liver genes involved in lipid metabolism after Δ6abc/5mutation. a Expression changes of genes in Log2 fold change between Δ6abc/5MT and WT salmon. Differentially expressed genes (DEGs, q < 0.05 & |log2FC| > 0.5) are labelled, except three genes with asterix (*) which had high log2 fold change but not significant (q > 0.05) b Correlation between gene expression and DHA content in phospholipid. Three fish individuals of each diet (plant oil or fish oil) and genetic (WT or Δ6abc/5MT) group were included in the analysis. Data of DHA measurement was acquired from Datsomor et.al, 2019
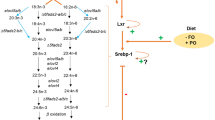
By comparing salmon gene promoter sequences to 6 transcription factor binding sites databases (CISBP, HUMAN.H10MO.B, HT-SELEX2, HumanTF, JASPAR, TRANSFAC), we identified 235 lipid metabolism genes with potential sterol regulatory elements (SRE), the Srebp binding sites, between 1000 bp upstream to 200 bp downstream from transcription starting sites (Supplementary Table 2). This includes Δ5fads2, Δ6fads2-a, elovl5-a, elovl5-b and elovl2 which are the major genes in LC-HUFA synthesis pathway. A recent study showed that CRISPR/Cas9-mediated editing of elovl2 in salmon has increased transcription of srebp1, Δ6fads2 and Δ5fads2 genes together with decreased LC-HUFA content, supporting the regulation of fads2 genes by the Srebp-1 transcription regulator (Fig. 7) [18]. However, the salmon Srebp-1 transcription factor is unlikely to induce expression of elovl5 and elovl2. This was because the expression of both elovl genes were stable in Δ6abc/5Mt compared to WT salmon, though srebp1 expression was upregulated. The elovl5 genes were also stable in elovl2-mutated salmon [18]. One possible reason is that the SRE in promoter regions of elovl5 and elovl2 genes may be more efficient for binding Srebp-2 rather than Srebp-1 [34], or that other transcription factors such as liver X receptor (LXR) are responsible stimulation of elovl genes in salmon under a plant oil diet. On the other hand, mammalian SREBP-1 can target both fatty acid desaturase (FADS2) and elongase (ELVOL5) genes and regulate LC-HUFA synthesis [35, 36].
To further investigate the relationship between key transcription factors and lipid metabolism genes, we compared the expression changes of the 230 lipid metabolism genes except LC-HUFA synthesis genes, either between mutated and WT salmon fed plant oil, or between mutated and WT salmon fed fish oil, or between WT salmon fed plant oil and fish oil (Fig. 6a). Several agpat3 and acsbg genes were significantly (q < 0.05 & |log2FC| > 0.5) up-regulated in plant oil mutated salmon together with up-regulated srebp1. The function of the Srebp-1 transcription factor in salmon is likely similar to its function in mammals, which works as a key transcription factor for hepatic lipogenesis, and agpat3 and acsbg genes are likely the key target genes of salmon Srebp-1. The same acsbg, agpat3 and srebp1 genes were also up-regulated when the elovl2 gene was CRISPR-mutated in salmon, confirming an increase of fatty acid acylation and lipogenesis in response to decreased tissue DHA content (Fig. 7) [18]. Other typical mammalian SREBP-1 targets, fasn, acc1 and elovl6 genes of fatty acid synthesis and elongation pathways were also up-regulated, but not significantly (q > 0.05) in mutated salmon compared to WT under the plant oil diet (Fig. 6). However, the transcriptional increase of these genes was much higher and significant (q < 0.05) in WT salmon fed the plant oil diet compared to fish oil. This means that the genes of fatty acid synthesis and elongation in salmon were not merely targeted by Srebp-1, but by other transcription factors, likely Srebp-2 [32] or Ppar-γ [37]. Genes of cholesterol metabolism including hmgcrab, mvd-a and sqlea-a were only highly up-regualted in WT fed plant oil diet versus fish oil, while no transcription change was observed in Δ6abc/5Mt versus WT salmon. Several studies have found up-regulation of cholesterol biosynthesis and srebp2 genes in salmon fed plant oils [12, 13, 15]. The present study has supported that the relationship between srebp2 and cholesterol biosynthesis genes is quite conserved in salmon as in mammals, and suggests that the SREBP binding sites of cholesterol biosynthesis genes were srebp2-specific (Fig. 7) [32].
CRISPR/Cas9-mediated mutation of fads2 genes in Δ6abc/5 also affected the fatty acid β-oxidation pathway in salmon. This was indicated by a strong down-regulation of acc2 gene following Δ6abc/5Mt (Fig. 5). Unlike the acc1 gene which is mostly involved in de-novo fatty acid synthesis in the cytosol, the acc2 gene in mammals produces mitochondria-associated malonyl-CoA which is a negative regulator of CPT1 and inhibits mitochondria β-oxidation [38, 39]. Therefore, the down-regualtion of acc2 in Δ6abc/5Mt salmon could suggest an increased fatty acid β-oxidation after disrutpion of LC-HUFA sythetic pathway. This could be regulated by PPAR which is key regualtor of fatty acid catabolism [28]. Similar to srebp1, we also found a negative correlation between DHA level and two ppara-a genes, though their expression levels were not changed after Δ6abc/5 mutation. As PUFA and their derivatives are known natural ligands of PPAR, the activation of PPAR and their target genes including fatty acid β-oxidation may not rely on increased transcirption of PPAR genes [40]. The increased β-oxidation was probably due to accumulation of 18:3n-3, 18:2n-6, and other intermediate fatty acids in the LC-HUFA synthesis pathway which cannot be synthesised further to DHA and EPA after disruption of fads2 genes. These fatty acids were most likely consumed alternatively in β-oxidation which was activated by the PPAR transcription factor [22]. Feeding of plant oil diets also induced cpt1a and abcd1, which are key genes involved in import of fatty acids into mitochondria and peroxisomes for catabolism (Fig. 7). However, a paralog gene cpt1b was down-regulated both after fads2-mutation and feeding plant oil diet. The reason for the down-regualtion is unclear and whether it would affect fatty acid β-oxidation needs to be further investigated. One possible explanation is that malonyl-CoA produced by acc1 or acc2 is less organelle-specific in salmon, and that the cpt1b gene could be inhibited by malonyl-CoA produced by acc1 in de-novo fatty acid synthesis.
Conclusions
CRISPR-Cas9 can be employed efficiently to mutate multiple fads2 genes simultaneously in salmon. However, mosaic effects are common, embodied by different indels among tissues and individuals. Exon skipping found in the Δ6fads2-a gene during transcription was predicted to result in the production of truncated proteins and strengthen the CRISPR-induced disruption of LC-HUFA synthesis in Δ6abc/5Mt salmon. Down-regulation of the targeted Δ5fads2, Δ6fads2-a and Δ6fads2-b genes were found in liver, which likely cause a decrease of LC-HUFA synthesis. On the other hand, the transcription of elovl5a, elovl5b and elovl2 genes in the LC-HUFA synthesis pathway was not affected. Since srebp1 genes were up-regulated in Δ6abc/5-mutated salmon the elovl genes were not likely regulated by this transcription factor. Increased de-novo fatty acid synthesis and lipogenesis was observed after Δ6abc/5Mt and could also be regulated by SREBP1. In addition, the level of transcriptional changes of fasn and acc1 genes involved in fatty acid synthesis were much higher when the fish was fed plant oil as compared to fish oil. This suggests that these genes were regulated by one or more transcriptional factors in addition to SREBP1. PPAR or SREBP2 are likely candidates. Increased fatty acid β-oxidation was also observed after Δ6abc/5Mt and was likely regulated by PPAR. The CRISPR-mutation of Δ6bcMt genes surprisingly revealed over 3000 DEGs in liver of salmon, and the DEGs were not enriched in any lipid metabolism pathways. The reason for the high number of DEGs in Δ6bcMt salmon was unclear and needs to be further investigated.
Methods
Generation of CRISPR/Cas9-mediated mutated salmon and feeding experiment
The generation of CRISPR/Cas9-mediated mutated salmon and the corresponding feeding trial was previously published in [22]. In brief, two types of fads2 mutants were generated with CRISPR/Cas9 injection into embryos, sperm and eggs were provided by AquaGen (Trondheim, Norway). Both times a single CRISPR guide RNA (gRNA) was used to target different combinations of fads2 genes simultaneously: A Δ6abc/5-mutated (Δ6abc/5Mt) salmon strain was generated using a gRNA targeting Δ6fads2-a (NCBI Gene ID 100136441), Δ6fads2-b (100329172), Δ6fads2-c (106584797) and Δ5fads2 (100136383). A Δ6bc-mutated (Δ6bcMt) salmon strain was generated targeting Δ6fads2-b and Δ6fads2-c. Both strains were co-injected with a gRNA targeting the slc45a2 (NCBI Gene ID gene 106563596), involved in melanin synthesis [19]. Target sequences of gRNAs were published in Datsomor et.al, 2019.
The feeding trial was performed on Atlantic salmon parr (N = 108) of approximately 85 ± 25 g for Δ6abc/5Mt salmon (N = 36), 104 ± 25 g for Δ6bcMt salmon (N = 36), and 176 ± 34 g for wildtype controls (WT; N = 36) at the Institute of Marine Research (Matre, Norway). Fish were initially fed a standard commercial diet until start of the experiment. A total of six experimental tanks were used with a common-garden approach, each containing 18 fish consisting of 6 Pit-tagged fish of the Δ6abc/5Mt, Δ6bcMt and WT. Three tanks were then fed a plant oil diet containing 5% LC-HUFA of total fatty acids, while the remaining three tanks were fed a fish oil diet with 20% LC-HUFA. The fatty acid composition of the diets was shown in detail in [22]. After 54 days of feeding, fish under plant oil diet reached 203 ± 51 g for Δ6abc/5Mt salmon, 281 ± 52 g for Δ6bcMt salmon and 250 ± 62 for WT, while the fish under fish oil diet reached 171 ± 36 g, 191 ± 69 g and 241 ± 47 g for the three groups respectively. Liver and muscle tissues from 6 fish per dietary treatment/strain were then sampled and tissues were flash frozen on dry ice and subsequently stored at − 80 °C. During tissue sampling, unnecessary pain was avoided by anesthetizing all fish by placing in freshwater containing 100 mg/L Finquel MS-222 (Tricaine Methanesulfonate) buffered with 100 mg/L sodium bicarbonate (Scan Vacc AS, Hvam, Norway) which caused rapid loss of consciousness (no body or opercula movement), this was followed by euthanasia using a blow to the head.
AmpliSeq
To confirm CRISPR/Cas9-induced mutations, AmpliSeq was conducted according to the Illumina protocol (16S Metagenomic Sequencing Library Preparation # 15044223 Rev. B, Illumina AS, San Diego, CA, USA). DNA was isolated from selected individuals from both liver and muscle using DNeasy blood and tissue kits (Qiagen, Hilden, Germany). Primers were designed to specifically amplify the regions around the CRISPR gRNA target sites (Table 1). For each sample the amplicons were generated in singleplex reactions, pooled and then purified using AMPure beads (Beckman Coulter Life Sciences, Indianapolis, IN, USA) before running index-PCR using the Nextera XT Index Kit (Illumina AS, San Diego, CA, USA). AmpliSeq libraries were subsequently normalized before sequencing the libraries as 300 bp paired-end reads on Illumina MiSeq (Illumina, San Diego, CA, USA) at Centre of Integrative Genetics (CIGENE, Ås, Norway). Raw .fastq reads were quality trimmed using cutdapt [41] before aligning them to the salmon genome ICSASG_v2 (Accession Number GCF_000233375.1, available for download at NCBI database https://www.ncbi.nlm.nih.gov/assembly/GCF_000233375.1/) using bwa mem [42] and saving files in .bam format. For each sample the proportion of indels for each base in a 25 bp window around the target sites was determined using the python3 coverage.py (https://gitlab.com/fabian.grammes/crispr-indel). Additionally we predicted the effect of each indel on the main transcript/protein using SnpEff [43].
RNA extraction and library preparation
Total RNA was extracted from liver of 36 individual fish by using RNeasy Plus Universal Mini kit (Qiagen AS, Hilden, Germany), according to manufacturer’s instruction. The 36 fish comprised 6 fish by group (strain by dietary treatment; two fish / tank). The RNA concentration and quality were assessed by Nanodrop 8000 (Thermo Scientific, Wilmington, USA) and Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). All samples had RIN values > 8.5. RNA-seq libraries were prepared using TruSeq Stranded mRNA Library Prep Kit (Illumina AS, San Diego, CA, USA). The libraries were subsequently sequenced using 100 bp single-end high-throughput mRNA sequencing (RNA-seq) on an Illumina Hiseq 2500 (Illumina AS, San Diego, CA, USA) at Norwegian Sequencing Centre (Oslo, Norway).
Data analysis and statistics
Read sequences were processed using the bcbio-nextgen pipeline (https://github.com/bcbio/bcbio-nextgen). In brief reads were aligned to the salmon genome (ICSASG_v2) using STAR [44]. The resulting .bam files were subsequently used to generate i) raw gene counts using featureCounts (v1.4.4) [45] using the NCBI Salmo salar Annotation Release 100 (available for download at https://ftp.ncbi.nlm.nih.gov/genomes/all/annotation_releases/8030/100/). ii) exon counts using DEXSeq (dexseq_count.py) [46]. In addition reads were mapped directly to the transcriptome using Salmon (v0.10.2) [47]. Gene IDs from NCBI GeneBank database (https://www.ncbi.nlm.nih.gov/) were used to identify genes in this study.
Expression analysis of the genes was performed using R (v3.4.1). Only genes with a minimum counts level of at least 1 count per million (CPM) in 75% of the samples were kept for further differential expression analysis (DEA). DEA was performed between groups (strain by dietary treatment, n = 6), using the generalized linear model (GLM) method in R package edgeR [48]. The present study focuses on three contrasts, Δ6abc/5-mutated salmon versus WT fed plant oil diet, Δ6abc/5-mutated salmon versus WT fed fish oil diet, and WT salmon fed plant oil versus fish oil diet. Genes with a false discovery rate (FDR), an adjusted p value (q) < 0.05 and absolute log2 fold change (|Log2FC|) > 0.5 were considered to be differentially expressed genes (DEGs) between the two test conditions. Subsequently, a KEGG ontology enrichment analysis (KOEA) was conducted using edgeR. A hypergeometric test was applied based on number of DEGs compared to total genes annotated to each KEGG pathway, and differences were considered significant when p < 0.005. All figures were made by using R package ggplot2 [49].
Availability of data and materials
Raw fastq files and raw gene counts table are publicly available under the accession: E-MTAB-8319 at the ArrayExpress Archive (https://www.ebi.ac.uk/arrayexpress/).
The R code which used to generate DEA and KOEA is publicly available at Fairdomhub (https://fairdomhub.org/investigations/242).
The protocol for 16S Metagenomic Sequencing Library Preparation is publicly available at Illumina (https://emea.support.illumina.com/downloads/16s_metagenomic_sequencing_library_preparation.html).
Gene IDs from NCBI GeneBank database (https://www.ncbi.nlm.nih.gov/) were used to identify genes in this study. For example, Δ6fads2-a 100136441 (https://www.ncbi.nlm.nih.gov/gene/100136441), Δ6fads2-b 100329172 (https://www.ncbi.nlm.nih.gov/gene/100329172), Δ6fads2-c 106584797 (https://www.ncbi.nlm.nih.gov/gene/106584797) and Δ5fads2 100136383 (https://www.ncbi.nlm.nih.gov/gene/100136383).
The salmon genome ICSASG_v2 (Accession Number GCF_000233375.1) is publicly available for download at NCBI database (https://www.ncbi.nlm.nih.gov/assembly/GCF_000233375.1/).
The Salmo salar Annotation Release for ICSASG_v2 (Annotation release ID 100) is used in this study and it is publicly available for download at NCBI database (https://ftp.ncbi.nlm.nih.gov/genomes/all/annotation_releases/8030/100/).
Abbreviations
- LC-HUFA:
-
Long-chain highly unsaturated fatty acids
- FADS2:
-
Fatty acid desaturases 2
- DEGs:
-
Differentially expressed genes
- SREBP1:
-
Sterol regulatory binding protein 1
- LXR:
-
Liver X receptor
- PPAR:
-
Peroxisome proliferator-activated receptor
- ELOVL5:
-
Elongase 5
- ELOVL2:
-
Elongase 2
- Δ5fad:
-
Δ5 fatty acid desaturase
- Δ6fad:
-
Δ6 fatty acid desaturase
- CPT1:
-
Carnitine palmitoyltransferase I
- ACC:
-
Acetyl-CoA carboxylase
- Indels:
-
Insertions and deletions
- gRNA:
-
Guide RNA
References
Ytrestøyl T, Aas TS, Åsgård T. Utilisation of feed resources in production of Atlantic salmon (Salmo salar) in Norway. Aquaculture. 2015;448:365–74. https://doi.org/10.1016/j.aquaculture.2015.06.023.
Sprague M, Dick JR, Tocher DR. Impact of sustainable feeds on omega-3 long-chain fatty acid levels in farmed Atlantic salmon, 2006-2015. Sci Rep. 2016;6:21892. https://doi.org/10.1038/srep21892.
Tocher DR. Metabolism and functions of lipids and fatty acids in teleost fish. Rev Fish Sci. 2003;11:107–84. https://doi.org/10.1080/713610925.
Bell JG, McEvoy J, Tocher DR, McGhee F, Campbell PJ, Sargent JR. Replacement of fish oil with rapeseed oil in diets of Atlantic salmon (Salmo salar) affects tissue lipid compositions and hepatocyte fatty acid metabolism. J Nutr. 2001;131:1535–43. https://doi.org/10.1093/jn/131.5.1535.
Gillard G, Harvey TN, Gjuvsland A, Jin Y, Thomassen M, Lien S, et al. Life-stage-associated remodelling of lipid metabolism regulation in Atlantic salmon. Mol Ecol. 2018;27:1200–13. https://doi.org/10.1111/mec.14533.
Horn SS, Ruyter B, Meuwissen THE, Hillestad B, Sonesson AK. Genetic effects of fatty acid composition in muscle of Atlantic salmon. Genet Sel Evol. 2018;50(1):23.
Monroig O, Zheng X, Morais S, Leaver MJ, Taggart JB, Tocher DR. Multiple genes for functional 6 fatty acyl desaturases (fad) in Atlantic salmon (Salmo salar L.): gene and cDNA characterization, functional expression, tissue distribution and nutritional regulation. Biochim Biophys Acta. 2010;1801:1072–81. https://doi.org/10.1016/j.bbalip.2010.04.007.
Morais S, Monroig O, Zheng X, Leaver MJ, Tocher DR. Highly unsaturated fatty acid synthesis in Atlantic salmon: characterization of ELOVL5- and ELOVL2-like elongases. Mar Biotechnol. 2009;11:627–39. https://doi.org/10.1007/s10126-009-9179-0.
Carmona-Antoñanzas G, Monroig O, Dick JR, Davie A, Tocher DR. Biosynthesis of very long-chain fatty acids (C>24) in Atlantic salmon: cloning, functional characterisation, and tissue distribution of an Elovl4 elongase. Comp Biochem Physiol B: Biochem Mol Biol. 2011;159:122–9. https://doi.org/10.1016/j.cbpb.2011.02.007.
Hastings N, Agaba MK, Tocher DR, Zheng X, Dickson CA, Dick JR, et al. Molecular cloning and functional characterization of fatty acyl desaturase and elongase cDNAs involved in the production of eicosapentaenoic and docosahexaenoic acids from alpha-linolenic acid in Atlantic salmon (Salmo salar). Mar Biotechnol. 2004;6:463–74. https://doi.org/10.1007/s10126-004-3002-8.
Zheng X, Tocher DR, Dickson CA, Bell JG, Teale AJ. Highly unsaturated fatty acid synthesis in vertebrates: new insights with the cloning and characterization of a delta6 desaturase of Atlantic salmon. Lipids. 2005;40:13–24. https://doi.org/10.1007/s11745-005-1355-7.
Jin Y, Olsen RE, Gillard GB, Østensen M-A, Korsvoll SA, Santi N, et al. A systemic study of lipid metabolism regulation in salmon fingerlings and early juveniles fed plant oil. Br J Nutr. 2018;120:653–64. https://doi.org/10.1017/S0007114518001885.
Leaver MJ, Villeneuve LA, Obach A, Jensen L, Bron JE, Tocher DR, et al. Functional genomics reveals increases in cholesterol biosynthetic genes and highly unsaturated fatty acid biosynthesis after dietary substitution of fish oil with vegetable oils in Atlantic salmon (Salmo salar). BMC Genomics. 2008;9:299. https://doi.org/10.1186/1471-2164-9-299.
Morais S, Pratoomyot J, Taggart JB, Bron JE, Guy DR, Bell JG, et al. Genotype-specific responses in Atlantic salmon (Salmo salar) subject to dietary fish oil replacement by vegetable oil: a liver transcriptomic analysis. BMC Genomics. 2011;12:255. https://doi.org/10.1186/1471-2164-12-255.
Liland NS, Espe M, Rosenlund G, Waagbø R, Hjelle JI, Lie Ø, et al. High levels of dietary phytosterols affect lipid metabolism and increase liver and plasma TAG in Atlantic salmon (Salmo salar L.). Br J Nutr. 2013;110:1958–67. https://doi.org/10.1017/S0007114513001347.
Nohturfft A, DeBose-Boyd RA, Scheek S, Goldstein JL, Brown MS. Sterols regulate cycling of SREBP cleavage-activating protein (SCAP) between endoplasmic reticulum and Golgi. Proc Natl Acad Sci U S A. 1999;96:11235–40. https://doi.org/10.1073/pnas.96.20.11235.
Szterk A, Roszko M, Sosińska E, Derewiaka D, Lewicki PP. Chemical composition and oxidative stability of selected plant oils. J Am Oil Chem Soc. 2010;87:637–45. https://doi.org/10.1007/s11746-009-1539-4.
Datsomor AK, Zic N, Li K, Olsen RE, Jin Y, Vik JO, et al. CRISPR/Cas9-mediated ablation of elovl2 in Atlantic salmon (Salmo salar L.) inhibits elongation of polyunsaturated fatty acids and induces Srebp-1 and target genes. Sci Rep. 2019;9:7533. https://doi.org/10.1038/s41598-019-43862-8.
Edvardsen RB, Leininger S, Kleppe L, Skaftnesmo KO, Wargelius A. Targeted mutagenesis in Atlantic salmon (Salmo salar L.) using the CRISPR/Cas9 system induces complete knockout individuals in the F0 generation. PLoS One. 2014;9:e108622. https://doi.org/10.1371/journal.pone.0108622.
Straume AH, Kjærner-Semb E, Ove Skaftnesmo K, Güralp H, Kleppe L, Wargelius A, et al. Indel locations are determined by template polarity in highly efficient in vivo CRISPR/Cas9-mediated HDR in Atlantic salmon. Sci Rep. 2020;10:409. https://doi.org/10.1038/s41598-019-57295-w.
Wargelius A, Leininger S, Skaftnesmo KO, Kleppe L, Andersson E, Taranger GL, et al. Dnd knockout ablates germ cells and demonstrates germ cell independent sex differentiation in Atlantic salmon. Sci Rep. 2016;6:21284. https://doi.org/10.1038/srep21284.
Datsomor AK, Olsen RE, Zic N, Madaro A, Bones AM, Edvardsen RB, et al. CRISPR/Cas9-mediated editing of Δ5 and Δ6 desaturases impairs Δ8-desaturation and docosahexaenoic acid synthesis in Atlantic salmon (Salmo salar L.). Sci Rep. 2019;9:16888. https://doi.org/10.1038/s41598-019-53316-w.
Kapahnke M, Banning A, Tikkanen R. Random splicing of several exons caused by a single base change in the target exon of crispr/cas9 mediated gene knockout. Cells. 2016;5. https://doi.org/10.3390/cells5040045.
Mou H, Smith JL, Peng L, Yin H, Moore J, Zhang X-O, et al. CRISPR/Cas9-mediated genome editing induces exon skipping by alternative splicing or exon deletion. Genome Biol. 2017;18:108. https://doi.org/10.1186/s13059-017-1237-8.
Prykhozhij SV, Steele SL, Razaghi B, Berman JN. A rapid and effective method for screening, sequencing and reporter verification of engineered frameshift mutations in zebrafish. Dis Model Mech. 2017;10:811–22. https://doi.org/10.1242/dmm.026765.
Sharpe JJ, Cooper TA. Unexpected consequences: exon skipping caused by CRISPR-generated mutations. Genome Biol. 2017;18:109. https://doi.org/10.1186/s13059-017-1240-0.
Popp MW, Maquat LE. Leveraging rules of nonsense-mediated mRNA decay for genome engineering and personalized medicine. Cell. 2016;165:1319–22. https://doi.org/10.1016/j.cell.2016.05.053.
Varga T, Czimmerer Z, Nagy L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim Biophys Acta. 1812;2011:1007–22. https://doi.org/10.1016/j.bbadis.2011.02.014.
Choi CS, Savage DB, Abu-Elheiga L, Liu Z-X, Kim S, Kulkarni A, et al. Continuous fat oxidation in acetyl-CoA carboxylase 2 knockout mice increases total energy expenditure, reduces fat mass, and improves insulin sensitivity. Proc Natl Acad Sci U S A. 2007;104:16480–5. https://doi.org/10.1073/pnas.0706794104.
Morais S, Taggart JB, Guy DR, Bell JG, Tocher DR. Hepatic transcriptome analysis of inter-family variability in flesh n-3 long-chain polyunsaturated fatty acid content in Atlantic salmon. BMC Genomics. 2012;13:410. https://doi.org/10.1186/1471-2164-13-410.
Tocher DR. Omega-3 long-chain polyunsaturated fatty acids and aquaculture in perspective. Aquaculture. 2015;449:94–107. https://doi.org/10.1016/j.aquaculture.2015.01.010.
Amemiya-Kudo M, Shimano H, Hasty AH, Yahagi N, Yoshikawa T, Matsuzaka T, et al. Transcriptional activities of nuclear SREBP-1a, −1c, and −2 to different target promoters of lipogenic and cholesterogenic genes. J Lipid Res. 2002;43:1220–35.
Carmona-Antoñanzas G, Tocher DR, Martinez-Rubio L, Leaver MJ. Conservation of lipid metabolic gene transcriptional regulatory networks in fish and mammals. Gene. 2014;534:1–9. https://doi.org/10.1016/j.gene.2013.10.040.
Carmona-Antoñanzas G, Zheng X, Tocher DR, Leaver MJ. Regulatory divergence of homeologous Atlantic salmon elovl5 genes following the salmonid-specific whole-genome duplication. Gene. 2016;591:34–42. https://doi.org/10.1016/j.gene.2016.06.056.
Matsuzaka T, Shimano H, Yahagi N, Amemiya-Kudo M, Yoshikawa T, Hasty AH, et al. Dual regulation of mouse Delta(5)- and Delta(6)-desaturase gene expression by SREBP-1 and PPARalpha. J Lipid Res. 2002;43:107–14.
Qin Y, Dalen KT, Gustafsson J-A, Nebb HI. Regulation of hepatic fatty acid elongase 5 by LXRalpha-SREBP-1c. Biochim Biophys Acta. 1791;2009:140–7. https://doi.org/10.1016/j.bbalip.2008.12.003.
Coleman RA, Lee DP. Enzymes of triacylglycerol synthesis and their regulation. Prog Lipid Res. 2004;43:134–76. https://doi.org/10.1016/S0163-7827(03)00051-1.
Abu-Elheiga L, Matzuk MM, Abo-Hashema KA, Wakil SJ. Continuous fatty acid oxidation and reduced fat storage in mice lacking acetyl-CoA carboxylase 2. Science. 2001;291:2613–6. https://doi.org/10.1126/science.1056843.
Mao J, DeMayo FJ, Li H, Abu-Elheiga L, Gu Z, Shaikenov TE, et al. Liver-specific deletion of acetyl-CoA carboxylase 1 reduces hepatic triglyceride accumulation without affecting glucose homeostasis. Proc Natl Acad Sci U S A. 2006;103:8552–7. https://doi.org/10.1073/pnas.0603115103.
Kliewer SA, Sundseth SS, Jones SA, Brown PJ, Wisely GB, Koble CS, et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors alpha and gamma. Proc Natl Acad Sci U S A. 1997;94:4318–23. https://doi.org/10.1073/pnas.94.9.4318.
Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011;17:10. https://doi.org/10.14806/ej.17.1.200.
Li H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv; 2013.
Cingolani P, Platts A, Wang LL, Coon M, Nguyen T, Wang L, et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff. Fly (Austin). 2012;6:80–92.
Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. https://doi.org/10.1093/bioinformatics/bts635.
Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–30. https://doi.org/10.1093/bioinformatics/btt656.
Anders S, Reyes A, Huber W. Detecting differential usage of exons from RNA-seq data. Genome Res. 2012;22:2008–17. https://doi.org/10.1101/gr.133744.111.
Patro R, Duggal G, Love MI, Irizarry RA, Kingsford C. Salmon provides fast and bias-aware quantification of transcript expression. Nat Methods. 2017;14:417–9. https://doi.org/10.1038/nmeth.4197.
Robinson MD, McCarthy DJ, Smyth GK. edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–40. https://doi.org/10.1093/bioinformatics/btp616.
Wickham H. ggplot2 - elegant graphics for data analysis. New York, NY: Springer-Verlag New York; 2016. https://doi.org/10.1007/978-0-387-98141-3.
Acknowledgements
We would like to thank Matilde Mengkrog Holen and Centre for Integrative Genetics (CIGENE) for help during RNA-Seq sample preparation.
Funding
The study was financed by the Norwegian Research Council project: “Towards the Digital Salmon: From a reactive to a pre-emptive research strategy in aquaculture (DigiSal)”. Grant number: 248792/O30. The funding agency played no part in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualization: YJ, AD, RE, JT, PW, FG, REO. Data Curation: YJ, FG. Formal Analysis: YJ, FG. Funding Acquisition: RE, JT, PW, JOV, AW. Methodology: YJ, FG. Resources: AD, RE, AW, PW, FG, REO. Visualization: YJ, FG. Writing – Original Draft Preparation: YJ, FG. Writing – Review & Editing: YJ, JOV, AW, AD, RE, JT, PW, FG, REO. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All experiments on animals were performed in strict accordance with the Norwegian Animal Welfare Act of 19th of June 2009. Experiments carried out in this study were approved by the Norwegian Animal Research Authority (NARA 5741).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Jin, Y., Datsomor, A.K., Olsen, R.E. et al. Targeted mutagenesis of ∆5 and ∆6 fatty acyl desaturases induce dysregulation of lipid metabolism in Atlantic salmon (Salmo salar). BMC Genomics 21, 805 (2020). https://doi.org/10.1186/s12864-020-07218-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-020-07218-1