Abstract
Background
The Russian White is a gene pool breed, registered in 1953 after crossing White Leghorns with local populations and, for 50 years, selected for cold tolerance and high egg production (EL). The breed has great potential in meeting demands of local food producers, commercial farmers and biotechnology sector of specific pathogen-free (SPF) eggs, the former valuing the breed for its egg weight (EW), EL, age at first egg (AFE), body weight (BW), and the latter for its yield of extraembryonic fluid (YEF) in 12.5-day embryos, ratio of extraembryonic fluid to egg weight, and embryo mass. Moreover, its cold tolerance has been presumably associated with day-old chick down colour (DOCDC) – white rather than yellow, the genetic basis of these traits being however poorly understood.
Results
We undertook genome-wide association studies (GWASs) for eight performance traits using single nucleotide polymorphism (SNP) genotyping of 146 birds and an Illumina 60KBeadChip. Several suggestive associations (p < 5.16*10− 5) were found for YEF, AFE, BW and EW. Moreover, on chromosome 2, an association with the white DOCDC was found where there is an linkage disequilibrium block of SNPs including genes that are responsible not for colour, but for immune resistance.
Conclusions
The obtained GWAS data can be used to explore the genetics of immunity and carry out selection for increasing YEF for SPF eggs production.
Similar content being viewed by others
Background
Programmes for the conservation of genetic resources including local, rare and endangered breeds are gaining increasing importance for the preservation of biodiversity, especially in farm animals (e. g., [1, 2]). Such gene pool chicken breeds are characterised by variations in phenotypic diversity and, in comparison to commercial poultry, are distinguished by high viability, special quality of meat and eggs, and/or having unique genetic features and appearance [1, 3,4,5,6]. One such breed is the Russian White (RW), whose development began around 1929 from crossing local chickens with parental stocks of the White Leghorn breed imported from Denmark, the UK and USA [7] in the Pyatigorsk and Kuchino breeding centres. Before RW was approved as a genuine breed in 1953, more attention was paid in Pyatigorsk to increase egg production (EL), and in Kuchino to increase body weight (BW).
The RW population maintained at the Russian Research Institute of Farm Animal Genetics and Breeding (RRIFAGB) was put under strong selection pressure for 50 years (1953 to 2003) [3, 7, 8]. Derived from one founder, its breeding was carried out selecting for tolerance to cold in chicks [7]. Initially, the day-old chick down colour (DOCDC) was yellow but selection for cold tolerance led to individuals with white down [3], the trait being supposedly controlled by the recessive gene sw for snow-white down in the chick [9]. It is currently used to develop poultry lines for the production of viral vaccines in embryonated eggs. The use of developing chick embryos makes it possible to increase the vaccine production and to expand the spectrum of viruses cultivated in the laboratory.
Despite the importance of this breed for all the above reasons, there is little or no information on the factors affecting (a) the yield of allanto-amniotic fluid that serves as a raw material for the vaccine production, and (b) as a consequence, the viral antibody titre in this fluid [10,11,12]. To carry out effective selection for increasing the yield of extraembryonic fluid (YEF), the genetic factors affecting this parameter must be established. Furthermore, in breeding egg laying hens, there are certain economically relevant traits at extreme ends of the phenotypic spectrum, knowledge of the genetics of which could be of use to breeders. These include AFE, egg weight (EW) and BW, and to take these traits into account is determinative both in the production of biopreparations and in the use of eggs for food purposes.
Recently, with the development and application of high-throughput genotyping technologies, it has become possible to identify associations of genomic regions and loci with selected traits. Traditional approaches, such as microsatellites randomly distributed throughout the genome, previously found quantitative trait loci (QTLs) for many economically important traits [13,14,15,16,17,18,19,20]. Locating single nucleotide polymorphisms (SNPs) in functional and positional candidate genes allowed testing for associations with the trait itself [21,22,23,24,25,26,27,28,29,30]. With the advent of whole genome next generation sequencing, however, either through the intermediate of a SNP chip, or more recently (as technology has become cheaper) by sequencing individuals directly, genome-wide association studies (GWASs) are discovering new loci associated with specific traits [31,32,33,34].
In particular, SNP genotyping using the Illumina Chicken 60 K SNP iSelect BeadChip (Illumina, USA), along with whole genomic sequencing, has found associations with traits related to poultry immunity and phenotypic characteristics [35, 36]. Given the importance of gene pool lines in general and the ability to refine pre-existing genotype × phenotype associations by GWAS, such studies are essential. Taking into account the pivotal importance of the RW line specifically for the vaccine and foodstuff production as well as understanding the genetic basis of cold tolerance, GWAS using RW seems to be a priority. With this in mind, the aim of this study was to establish hitherto undiscovered associations in the genome of Russian gene pool chickens with the following traits: EW, YEF, ratio of extraembryonic fluid to egg weight (REFEW), embryo mass (EM), EL, AFE, BW and, because of its association with cold tolerance, DOCDC.
Results
Day-old chick down colour
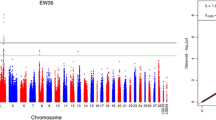
As a result of the genotyping of 146 RW females from 12 sires (Table 1) using 35,390 SNPs spread across 28 autosomes (Additional file 1: Table S1), five markers located on the chicken chromosome (GGA) 2 region were detected to be suggestively associated (p ≤ 4.2e-5) with DOCDC. The phenotypic variance explained by the set of SNPs was 11% (SE = 0.12). Three SNPs on GGA4 (rs16455118), GGA7 (rs317256404) and GGA28 (rs16209462) were also above suggestive line, with the phenotypic variance explained by them being 33% (SE = 0.18). The Manhattan and quantile-quantile (Q-Q) plots for DOCDC are presented in Fig. 1, and detailed information about the appropriate associated markers is given in Table 2. The observed genomic inflation factor (λg) was 1.019.
The level of linkage disequilibrium (LD) on GGA2 was calculated for the region span ranging between 119.6 to 124.4 Mb (Fig. 2). The region contained 123 SNPs, five of which were suggestively associated with DOCDC. Only two LD blocks from 24 detected ones were selected for the further detailed analysis. The first 11.8-kb haplotype block bordered on the most suggestive SNP (rs314384321), and included the genes LRRCC1 (leucine rich repeat and coiled-coil centrosomal protein 1) and SLC7A13 (solute carrier family 7 member 13). The second 180.3-kb block, with the suggestive SNP rs16116752 inside, contained the ZFHX4 (zinc finger homeobox 4) gene as well as two uncategorised non-coding RNA (ncRNA) loci, LOC107052753 and LOC101749450. One suggestive SNP each was also found in the genes DYSF (dysferlin) on GGA4 (rs16455118), PLEKHM3 (pleckstrin homology domain containing M3) on GGA7 (rs317256404) and SPPL2B (signal peptide peptidase like 2B) on GGA28 (rs16209462).
Yield of extraembryonic fluid
Two regions locating on GGA2 between 121.4 and 121.8 Mb and on GGA12 between 0.9 and 1.2 Mb were detected to be associated with YEF (Table 2). On GGA2, the SNP rs13730111 passed the significant test (p = 1.24e-6), while rs316856766 passed the suggestive line (p = 1.4e-5). Estimated explained phenotypic variance for both markers was 17.9% (SE = 0.20). Suggestive association on GGA12 was detected for two markers, rs15630281 and rs315166929, with explained phenotypic variance being 10.6% (SE = 0.12). The respective Manhattan and Q-Q plots are shown in Fig. 1, with the observed λg being equal to 0.93.
On GGA2, the significant SNP rs13730111 was located in the ZNF704 (zinc finger protein 704) gene, and the CA2 (carbonic anhydrase 2) gene neighboured the suggestive SNP rs316856766. On GGA12, LD analysis was performed for region 0.9 to 1.2 Mb (Fig. 3), and resulted in identifying the SNP rs315166929 in LD block of 27 kb and another SNP, rs15630281, between two haplotype blocks. The whole region contained a single gene, RFT1 (RFT1 homolog), and the closest described gene, PRKCD (protein kinase C delta), situated 60 kb aside 25-kb LD block with high LD status (r2 > 0.75).
Age at first egg
Only one marker (rs317931060) on GGA1 passed the suggestive line for AFE (Table 2), with p-value = 1.5e-5 and 13% (SE = 0.17) of phenotypic variance explained. Despite a low signal, we detected a quite remarkable peak below the suggestive marker line on GGA1 (λg = 0.995; Fig. 1). LD analysis was performed for 8-Mb region containing suggestive and peak SNPs with p ≤ 8.18e-4 (Fig. 4). Among three identified blocks, a suggestive SNP was located in a block with the lowest LD status as compared to others. The genes nearest to the suggestive 36-kb region were DYNC2H1 (dynein cytoplasmic 2 heavy chain 1) and PDGFD (platelet derived growth factor D). We also noted a region with a high LD, containing the SNP rs13558365 and coinciding with the DCUN1D5 (defective in cullin neddylation 1 domain containing 5) gene.
Body weight
For BW, no SNPs were detected to be significantly associated with this trait. A single SNP, rs15619223, on GGA4 passed the suggestive line (p = 2.9e-5; λg = 1.019; Table 2 and Fig. 1), explaining 10.5% (SE = 0.14) of phenotypic variance. The marker was located within the LCORL (ligand dependent nuclear receptor corepressor like) gene.
Egg weight
For EW, two suggestively associated SNPs were identified on GGA2 (Table 2), with the total explained phenotypic variance being 13% (SE = 0.14). These two SNPs, rs14201361 and rs14200974, were presented in the genes KIAA1468 (lisH domain and HEAT repeat-containing protein) and PHLPP1 (PH domain and leucine rich repeat protein phosphatase 1), respectively. Also, there was the ZCCHC2 (zinc finger CCHC-type containing 2) gene between two SNPs in a region 68.3 to 68.5 Mb. One SNP on GGA4 was located on the suggestive line with p-value = 5.42e-5 and within the TLL1 (tolloid-like protein 1 precursor) gene. The Manhattan and Q-Q plots for EW are presented in Fig. 1, with the observed λg being equal to 1.0106.
Other traits
We did not detect any significant or suggestive associations for EM, EL and REFEW. The markers for EM and EL that had the greatest p-values were rs10724110 (p = 1e-4) on GGA2 and rs317565390 (p = 9.6e-5) on GGA8, respectively. Both markers are located in intergenic regions and have no links with any known genes. For REFEW, we identified the markers rs14196507 and rs13730111 on GGA2, rs315166929 on GGA12, and rs15763249 on GGA15 (p = 6.3*10–5 to 7.9*10–5). The SNP rs13730111 was also described in the given study as a significant marker for YEF. The second marker on GGA2 (rs14196507) is located in an intronic region of the RREB1 (ras responsive element binding protein 1) gene. The markers on GGA12 and GGA15 are both located in intergenic regions with no link to known genes.
Also, we found genetic correlation between the pairs of traits EL and AFE, EL and EW, and EW and YEF that was respectively 0.48 ± 0.09, − 0.21 ± 0.05; and 0.82 ± 0.13 (p < 0.05).
Discussion
Day-old chick down colour
Typically, the down of RW chicks at day old is coloured in different shades of yellow. The first chicks with the white down were noticed during long-term selection for low temperature tolerance. Chicks with the white down were more tolerant to low temperatures and diseases, so breeders used colour as marker during the selection process [3]. Over the last decade, when research and selection process was ceased, white chicks still appeared in the progeny of the studied RW population. We found eight markers suggestively associated with DOCDC. The candidate genes located on GGA2 were LRRCC1 encoding a centrosomal protein that maintains the structural integrity of the centrosome and plays a key role in mitotic spindle formation, SLC7A13 encoding a protein mediating the transport L-aspartate and L-glutamate in a sodium-independent manner, and ZFHX4 encoding a protein potentially playing a part in neural and muscle differentiation. Two extra genes, LOC107052753 and LOC101749450, were uncategorised ncRNA loci. According to the Variant Effect Predictor (VEP) examination [37, 38], the SNPs rs15150566 and rs16116752 are intronic variants and modifiers of the genes RALYL and ZFHX4, respectively. The SNP rs14243963 is a synonymous variant meaning that has no impact on the IMPA1 gene function. The DYSF gene on GGA4 encodes a skeletal muscle protein that is involved in muscle contraction and binds caveolin-3, a skeletal muscle membrane protein, which is important in the formation of caveolae. The three other found SNPs, rs16455118, rs317256404 and rs16209462, are located within introns. The SPPL2B gene on GGA28 encodes a member of the GXGD proteases that are transmembrane proteins with two conserved catalytic motifs localized within the membrane-spanning regions. This enzyme localizes to endosomes, lysosomes, and the plasma membrane. It cleaves the transmembrane domain, which triggers cytokine expression in the innate and adaptive immunity pathways. The function of the protein encoded by the PLEKHM3 gene is characterized as metal ion binding and participation in skeletal muscle (myoblast) differentiation by acting as a scaffold protein for AKT1. None of the above genes may be directly related to pigmentation or DOCDC variants.
Thus, using a case-control GWAS approach for analysing phenotypes, we detected association with the genes responsible for immune system (SPPL2B) and muscle growth (LLRCC1 and ZFHX4) in chickens. In the study by Psifidi et al. [39], several associations with immune resistance were also found on GGA2, but 20 Mb away from the loci detected in our study. Other associations were determined on GGA20, GGA11 and GGA13 [39] as well as GGA1, GGA5, GGA16 and GGA24 [36]. Better understanding of possible correlation between cold tolerance and the white DOCDC can be achieved by more accurate phenotypic data recording. Renewed programme of strong selection for cold tolerance will facilitate an increase in the number of phenotyped and genotyped individuals to find correlation between two traits by using common statistical approaches as the first step in future studies. At the same time, growing number of the genotyped animals subject to phenotypic recording will also improve sensitivity of the GWAS analysis. The observed genomic inflation factor (λg) was close to 1, suggesting no population stratification. The Q-Q plot revealed a slight deviation from the distribution under the null hypothesis, which indicated a moderate association between SNPs and DOCDC.
Yield of extraembryonic fluid
This trait is highly important in the process of producing embryo vaccines. Specific pathogen-free (SPF) eggs having no antibodies or pathogens are widely used for producing vaccines for animals and humans. Increasing volume of extraembryonic fluid results in a higher virus titre meaning an essential economical effect for biological preparation producers. The observed average YEF (9.5 ml; Table 3) means that three eggs are required to produce a single vaccine dose. Industry demand in establishing domestic poultry stocks producing SPF eggs for internal market inspired the current research targeting YEF in the RW breed. Trait variation in generation F0 was 17%, and selection of birds based on increasing of YEF level reduce it to 9.8% in F3. We did not find any previous GWASs on extraembryonic fluid, although some papers [10, 11] discussed selection in chickens based on the allanto-amniotic fluid volume, suggesting that because of a limited number of SPF egg producers there might be a restricted amount of information available.
The present study revealed two SNPs on GGA2 significantly and suggestively associated with YEF. Both markers are located in intronic regions. Close browsing of the region showed that significant marker was included in the ZNF704 gene known to have expression in ovary and endometrium. The nearest to the suggestive SNP was the CA2 gene responsible for encoding a protein that catalyses reversible hydration of carbon dioxide. Other suggestive regions were detected on GGA12, containing RFT1 that is homologous to a yeast gene encoding an enzyme of the endoplasmic reticulum membrane in the pathway for the N-glycosylation of proteins, and the PRKCD gene encoding a protein from the kinase C family of serine- and threonine-specific protein kinases that is a positive regulator of the cell cycle progression and can positively or negatively regulate apoptosis. The PRKCD gene is expressed mainly in blood vessels and during their development, while one of the allantois functions is transport of oxygen, nutrients and excretion products, which is similar to the blood vessel system. The observed λg was lower than 1 and deviation of markers on the Q-Q plot was lower, suggesting a possible bias in GWAS.
In the study conducted by Psifidi et al. [39], several QTLs related to AFE and available in the Chicken QTLdb [40] were detected on GGA1, GGA2, GGA3, GGA4, GGA5, GGA7, GGA11, GGA13, GGA24 and GGAZ. A GWAS analysis of AFE presented by Yuan et al. [33] was based on White Leghorns and found suggestive associations on GGA16 and GGA23. Despite our expectations, we detected only one SNP suggestively associated with AFE on GGA1 and no significant associations. Within the SNP proximity we found three blocks with high LD structure. Two genes closest to an LD block that included the suggestive SNP were: DYNC2H1 responsible for a protein involved in retrograde transport in the cilium and playing a role in intraflagellar transport, a process required for ciliary/flagellar assembly, and PDGFD encoding protein member of the platelet-derived growth factor family. Third gene, DCUN1D5, was found in an adjacent LD block and plays a part in neddilation of the NEDD8 gene and SCF-complex (Skp, Cullin, F-box containing complex). Because λg was close to 1, no population stratification should be assumed. The Q-Q plot showed a slight deviation from the expected value, which indicated a moderate association between SNPs and AFE.
Body weight
Previous studies showed a suggestive association of BW with a single SNP on GGA4 [41, 42]. The SNP detected in our investigation was in the LCORL gene, polymorphisms in which are linked with measures of skeletal frame size and adult height. Some GWASs [39, 41, 43] showed significant associations of BW with markers on GGA4. SNPs and genes described in previous reports were not concordant with our study, although they suggested a quite a narrow region between 60 and 80 Mb on GGA4 using the same 60 K SNP chip [39, 41, 43]. Limited number of genotyped birds, small population and direct phenotypic data can be possible explanation of a weak signal of SNPs in the given study. Despite the absence of significant associations, we presume the LCORL gene as a possible candidate responsible for birds growing and body weight improvement. The observed values on the Q-Q plot had negative deviation from the expected values, and at the same time λg was close to 1. In this particular case, the GWAS led to an association that can be treated as underestimated.
Egg weight
Studies related to the association of EW with SNPs were done by Liao et al. [44] and Fan et al. [45]. Both studies focused on significant association with markers on GGA4, but several associations were also shown on GGA7, GGA3, GGA1 and GGA2. We found two suggestively associated SNPs that were 67.2 Mb away from rs14254270 on GGA2 published in the Fan et al. paper [45]. These were located within the PHLPP1 gene encoding a protein, which promotes apoptosis by dephosphorylating and inactivating the serine/threonine kinase and conventional/novel protein kinase C (PKC) isoforms, and KIAA1468 that participates in intracellular cholesterol transport. A single suggestive SNP was also detected on GGA4, and, as compared to previously shown results, the marker was situated 2 Mb aside from the published rs313911044 but close to ZCCHC2, with annotations related to this gene including nucleic acid binding and phosphatidylinositol binding. A high population stratification was shown by λg in EW, whereas the Q-Q plot reflected a low deviation of expected and observed markers.
Other traits
Although there were no significant or suggestive SNP associations for EM, EL and REFEW, we additionally established genetic correlation for few pairs of phenotypic traits. The observed correlation can be used as a ground for multi-trait GWAS as described in Turley et al. [46]. In particular, EL may be analysed along with YEF as these traits had a high genetic correlation (r = 0.48 ± 0.09, p < 0.05). The traits EW and YEF were highly correlated (r = 0.82 ± 0.13, p < 0.05) and should be analysed together, as well. A similar correlation coefficient between EW and the absolute YEF value (r = 0.71 ± 0.03, p < 0.01) was previously reported for the Russian White breed, while that between EW and the relative YEF value was 0.31 ± 0.06 (p < 0.01) [47]. At the same time, the found negative correlation between EL and EW (r = − 0.26 ± 0.05, p < 0.05) comparable to that in other studies (e.g. -0.37 ± 0.06, [48]) should be taking into consideration because of an expected interest of farmers to get large number of eggs during laying period.
Conclusions
Our study has represented the first GWAS analysis completed on Russian gene pool chickens. The significant and suggestive associations we found for YEF may serve as an important information for future allanto-amniotic fluid studies. The hypothetical association between the white DOCDC and cold tolerance in chicks will be investigated further and in more detail to provide a genetic source of adaptation in poultry. Studies on production traits provided essential information for future breed development and selection programme at the RRIFAGB Collective Use Centre ‘Genetic Collection of Rare and Endangered Chicken Breeds’ (CUC GCRECB). To assess potential of the found markers for marker-assisted selection (MAS), it would be desirable to perform a deeper analysis of suggestive regions using an expanded dataset and sequence information. The RW breed can be a valuable resource for local farmers and biotechnology sector, and the obtained data will further be used at the CUC GCRECB to select a breeding core for SPF eggs production.
In future studies, collection of additional phenotypic data and genotypes will be done for the RW breed, and MAS will be explored using SNPs that would explain a larger proportion of phenotypic variance. In addition, an extended GWAS can be done by using multi-trait analysis [46] for the correlated trait pairs identified in this study (EL–AFE, EL–EW and EW–YEF).
Methods
Samples and traits
Blood samples were collected from the wing vein of chickens by the standard venepuncture procedure. Animals were kept alive after blood collection and were not culled after the experiment. Genomic DNA was isolated from blood samples using a phenol/chloroform method [49]. DNA concentration ranged between 50 and 500 ng/μl. DNA quality and concentration corresponded to the requirements for the Illumina Infinium SNP genotyping platform.
The experimental chickens were a resource population of the RW breed maintained at the RRIFAGB CUC GCRECB. They were kept under the same conditions in individual cages and fed a commercial diet that contained 17% raw protein and 270 kcal energy per 100 g. In the current GWAS, 146 individuals representing progeny of 12 sires were tested. According to the pedigree, all animals descending from one sire were presented by half sibs (i.e. produced from different dams) and full sibs (from the same dam). The progeny data for each sire including number of half sibs, number of full sibs groups, mean number and standard deviation are given in Table 1. Eight animals with phenotypic records but unknown pedigree information were also included into the analysis.
The GWASs were performed using seven phenotypic traits: EW, YEF, REFEW, EM, EL, AFE, BW and DOCDC. For the EW, YEF and EM traits six sequentially laid eggs were determined per hen at age of 34 weeks. Liquid volume as a measure of YEF, and EM were found in 12.5-days embryos (see also Additional file 2). Total number of eggs (EL) was recorded during the egg laying period from first egg up to age of 52 weeks. BW of live hens was measured at age of 52 weeks. All data deviating by ±3 SD and more from a mean was excluded prior to analysis. DOCDC trait was recorded for genotyped animals by visual control on the first day after hatching. The case-control trait had two levels: white and yellow (Additional file 3: Figure S1).
Heritability estimates for EW, YEF and EL were computed by means of parent-offspring regression. Due to the lack of phenotypic records from parents for AFE and BW in the present study, their heritability values were derived from the data published by Niknafs et al. [50], while no heritability estimates were available for EM and REFEW. Descriptive statistics for quantitative traits used in association studies were calculated in RStudio [51]. Heritability and descriptive statistics are shown in Table 3.
Genotyping
Genotyping was performed in the GeneSeek, Inc. laboratory (Lincoln, NE, USA) using Illumina Chicken 60KBeadChip. Quality control was carried out using the Plink1.9 programme [52]. SNPs were removed if they did not pass the following criteria: the call rate was less than 95%, minor allele frequency was lower than 0.01, missing rate per SNP was more than 20%, and Hardy-Weinberg equilibrium probability was less than 1e-4. SNPs on the sex chromosome GGAZ as well as linkage groups LGE64 and LGE22 treated as chromosomes were also excluded from the analysis. Missing SNPs were imputed using the Beagle 4.1 software [53]. The final SNP dataset was presented by 28 autosomes and embraced 35,390 SNPs. Marker information per chromosome is summarised in Additional file 1: Table S1. Extremely high SNP density on GGA16 resulted from lower number of SNPs used and smaller chromosome length.
Whole genome association studies
A whole genome scan, with accounting of high relationship structure, was performed using a mixed model approach implemented in the Efficient Mixed-Model Association eXpedited (EMMAX) software [54]. SNP effect was computed using the following model: Y = Xb + u + e, where Y is a vector of phenotypes, b is a SNP effect, X is a design matrix of SNP genotypes, u is a vector of additive genetic effects assumed to be normally distributed with the mean equal to 0 and (co) variance \( {\upsigma}_a^2 \) G, with \( {\upsigma}_a^2 \) as the additive genetic variance and G as the genomic relationship matrix, and e is a vector of random residual effects. The genome-wide significance was assessed using the simpleM method [41, 55] in R [56], for calculation of effective number of independent tests, Meff . The significance and suggestive levels were set as 2.58*10− 6 (0.05/19,381) and 5.16*10− 5 (1.00/19,381), respectively. The Q-Q and Manhattan plots were derived from the GWAS results using the qqman package [57] within the R software. Genomic inflation factor (λg) was computed based on p-values from the GWAS analysis by determining a ration between the median of the resulting chi-squared test statistics and the expected median of the chi-squared distribution in the R software. Estimation of phenotypic variance and heritability using the genomic-relatedness-based restricted maximum-likelihood (GREML) method in family data [58] was done by means of the GCTA software [59], threshold level for off-diagonals (make-bK parameter) being set at 0.5 that corresponds to close relationships among animals.
The post GWAS analysis included the LD determination of the chromosomal regions with significant SNPs that was performed using PLINK and Big-LD R-package [60]. Ensembl genome database including Gallus_gallus-5.0 genome browser and VEP [37, 38], NCBI databases [61] and GeneCards [62] were used for getting information about SNPs and relevant gene annotation.
Abbreviations
- AFE:
-
Age at first egg
- bp:
-
Base pair
- BW:
-
Body weight
- CUC GCRECB:
-
Collective Use Centre ‘Genetic Collection of Rare and Endangered Chicken Breeds
- DOCDC:
-
Day-old chick down colour
- EL:
-
Egg production
- EM:
-
Embryo mass
- EW:
-
Egg weight
- GGA:
-
Chicken (Gallus gallus) chromosome
- GWAS:
-
Genome-wide association study
- kb:
-
Kilobase
- LD:
-
Linkage disequilibrium
- MAS:
-
Marker-assisted selection
- Mb:
-
Megabase
- ncRNA:
-
Non-coding RNA
- Q-Q:
-
Quantile-quantile
- QTL:
-
Quantitative trait locus
- REFEW:
-
Ratio of extraembryonic fluid to egg weight
- RRIFAGB:
-
Russian Research Institute of Farm Animal Genetics and Breeding
- RW:
-
Russian White
- SD:
-
Standard deviation
- SE:
-
Standard error
- SNP:
-
Single nucleotide polymorphism
- SPF:
-
Specific pathogen-free
- VEP:
-
Variant Effect Predictor
- YEF:
-
Yield of extraembryonic fluid
References
Weigend S, Romanov MN. The world watch list for domestic animal diversity in the context of conservation and utilisation of poultry biodiversity. Worlds Poult Sci J. 2002;58(4):519–38.
Food and Agriculture Organization of the United Nations. Food outlook: biannual report on global food markets, November 2017. Rome: Food and Agriculture Organization of the United Nations; 2017. p. 144p.
Sokolova AN. [Genetic and selection methods of creation of a chicken population with an increased resistance to neoplasms]. Author's abstract, Diss. Doct. Agr. Sci. St. Petersburg, Pushkin: RRIFAGB; 1999. 56 p.
Weigend S, Romanov MN. Current strategies for the assessment and evaluation of genetic diversity in chicken resources. Worlds Poult Sci J. 2001;57(3):275–88.
Soller M, Weigend S, Romanov MN, Dekkers JC, Lamont SJ. Strategies to assess structural variation in the chicken genome and its associations with biodiversity and biological performance. Poultry Sci. 2006;85(12):2061–78.
Yurchenko O, Makarova A, Karpukhina I, Vakhrameev A. Domestic breeds and populations of chickens. Zhivotnovod Ross. 2017;(2):7–10.
Paronyan IA, Yurchenko OP. Domestic fowl. In: Dmitriev NG, Ernst LK, editors. Animal genetic resources of the USSR. Rome: Food and Agriculture Organization of the United Nations; 1989. FAO Animal Production and Health Paper 65, ch. 13. p. 437–68.
Dementeva NV, Romanov MN, Kudinov AA, Mitrofanova OV, Stanishevskaya OI, Terletsky VP, Fedorova ES, Nikitkina EV, Plemyashov KV. Studying the structure of a gene pool population of the Russian white chicken breed by genome-wide SNP scan. Selskokhoziaĭstvennaia Biol. 2017;52(6):1166–74.
Hutt FB. Snow-white down in the chick. J Hered. 1951;42(3):117–20.
Tyapugin E. Selection of chicken embryos for the biological industry. Ptitsevodstvo (Moscow). 2012;(1):45–9.
Lapa MA. Influence of various factors on the volume of the allanto-amniotic fluid of the developing chick embryos. Izvestiya of the St. Petersburg State Agrarian Univ. 2014;37:65–8.
Lapa MA. Influence of the genotype of dams and sires, and the age of developing chick embryos on the volume and quality of the allanto-amniotic fluid. Genetika i razvedenie zhivotn. 2015;(1):14–20.
van Kaam JBCHM, van Arendonk JAM, Groenen MAM, Bovenhuis H, Vereijken ALJ, Crooijmans RPMA, van der Poel JJ, Veenendaal A. Whole genome scan for quantitative trait loci affecting body weight in chickens using a three generation design. Livest Prod Sci. 1998;54(2):133–50.
Tatsuda K, Fujinaka K. Genetic mapping of the QTL affecting body weight in chickens using a F2 family. Br Poult Sci. 2001;42(3):333–7.
Ikeobi CO, Woolliams JA, Morrice DR, Law A, Windsor D, Burt DW, Hocking PM. Quantitative trait loci for meat yield and muscle distribution in a broiler layer cross. Livest Prod Sci. 2004;87(2–3):143–51.
Zhou H, Deeb N, Evock-Clover CM, Ashwell CM, Lamont SJ. Genome-wide linkage analysis to identify chromosomal regions affecting phenotypic traits in the chicken. II Body composition. Poultry Sci. 2006;85(10):1712–21.
Ambo M, Moura AS, Ledur MC, Pinto LF, Baron EE, Ruy DC, Nones K, Campos RL, Boschiero C, Burt DW, Coutinho LL. Quantitative trait loci for performance traits in a broiler × layer cross. Anim Genet. 2009;40(2):200–8.
Goraga ZS, Nassar MK, Brockmann GA. Quantitative trait loci segregating in crosses between New Hampshire and white Leghorn chicken lines: I. Egg production traits. Anim Genet. 2012;43(2):183–9.
Nassar MK, Goraga ZS, Brockmann GA. Quantitative trait loci segregating in crosses between New Hampshire and white. Leghorn chicken lines: II. Muscle weight and carcass composition. Anim Genet. 2012;43(6):739–45.
Nassar MK, Goraga ZS, Brockmann GA. Quantitative trait loci segregating in crosses between New Hampshire and white Leghorn chicken lines: III. Fat deposition and intramuscular fat content. Anim Genet. 2013;44(1):62–8.
Dunn IC, Miao YW, Morris A, Romanov MN, Wilson PW, Waddington D. A study of association between genetic markers in candidate genes and reproductive traits in one generation of a commercial broiler breeder hen population. Heredity. 2004;92(2):128–34.
Shen X, Zeng H, Xie L, He J, Li J, Xie X, Luo C, Xu H, Zhou M, Nie Q, Zhang X. The GTPase activating rap/RanGAP domain-like 1 gene is associated with chicken reproductive traits. PLoS One. 2012;7(4):e33851.
Boschiero C, Jorge EC, Ninov K, Nones K, do Rosário MF, Coutinho LL, Ledur MC, Burt DW, Moura AS. Association of IGF1 and KDM5A polymorphisms with performance, fatness and carcass traits in chickens. J Appl Genet. 2013;54(1):103–12.
Felício AM, Boschiero C, Balieiro JC, Ledur MC, Ferraz JB, Moura AS, Coutinho LL. Polymorphisms in FGFBP1 and FGFBP2 genes associated with carcass and meat quality traits in chickens. Genet Mol Res. 2013;12(1):208–22.
Felício AM, Boschiero C, Balieiro JC, Ledur MC, Ferraz JB, Michelan Filho T, Moura AS, Coutinho LL. Identification and association of polymorphisms in CAPN1 and CAPN3 candidate genes related to performance and meat quality traits in chickens. Genet Mol Res. 2013;12(1):472–82.
Sheng Q, Cao D, Zhou Y, Lei Q, Han H, Li F, Lu Y, Wang C. Detection of SNPs in the cathepsin D gene and their association with yolk traits in chickens. PLoS One. 2013;8(2):e56656.
Pértille F, Zanella R, Felício AM, Ledur MC, Peixoto JO, Coutinho LL. Identification of polymorphisms associated with production traits on chicken (Gallus gallus) chromosome 4. Genet Mol Res. 2015;14(3):10717–28.
Dementeva NV, Mitrofanova OV, Tyshchenko VI, Terletskiy VP, Yakovlev AF. The rate of weight gain and productivity of a chicken broiler cross with various polymorphic types of the myostatin gene. Russ J Genet Appl Res. 2017;7(1):1–5.
Mitrofanova OV, Dementeva NV, Krutikova AA, Yurchenko OP, Vakhrameev AB, Terletskiy VP. Association of polymorphic variants in MSTN, PRL, and DRD2 genes with intensity of young animal growth in Pushkin breed chickens. Tsitol Genet. 2017;51(3):179–84.
Lee MO, Womack JE, Romanov MN, Plemyashov KV, Dementieva NV, Mitrofanova OV, Barkova OY. Haplotype structure and copy number polymorphism of the beta-defensin 7 genes in diverse chicken breeds. Anim Genet. 2017;48(4):490–2.
Qanbari S, Simianer H. Mapping signatures of positive selection in the genome of livestock. Livest Sci. 2014;166(1):133–43.
Mignon-Grasteau S, Rideau N, Gabriel I, Chantry-Darmon C, Boscher MY, Sellier N, Chabault M, Le Bihan-Duval E, Narcy A. Detection of QTL controlling feed efficiency and excretion in chickens fed a wheat-based diet. Genet Sel Evol. 2015;47:74.
Yuan J, Wang K, Yi G, Ma M, Dou T, Sun C, Qu LJ, Shen M, Qu L, Yang N. Genome-wide association studies for feed intake and efficiency in two laying periods of chickens. Genet Sel Evol. 2015;47:82.
Pértille F, Moreira GC, Zanella R, Nunes JR, Boschiero C, Rovadoscki GA, Mourão GB, Ledur MC, Coutinho LL. Genome-wide association study for performance traits in chickens using genotype by sequencing approach. Sci Rep. 2017;7:41748.
Sun Y, Liu R, Zhao G, Zheng M, Sun Y, Yu X, Li P, Wen J. Genome-wide linkage analysis identifies loci for physical appearance traits in chickens. G3 (Bethesda). 2015;5(10):2037–41.
Raeesi V, Ehsani A, Torshizi RV, Sargolzaei M, Masoudi AA, Dideban R. Genome-wide association study of cell-mediated immune response in chicken. J Anim Breed Genet. 2017;134(5):405–11.
Chicken (Gallus_gallus-5.0). Ensembl, EMBL-EBI. 2016. http://www.ensembl.org/Gallus_gallus/Info/Index. Accessed 10 Nov 2018.
Zerbino DR, Achuthan P, Akanni W, Amode MR, Barrell D, Bhai J, Billis K, Cummins C, Gall A, Girón CG, Gil L, Gordon L, Haggerty L, Haskell E, Hourlier T, Izuogu OG, Janacek SH, Juettemann T, To JK, Laird MR, Lavidas I, Liu Z, Loveland JE, Maurel T, McLaren W, Moore B, Mudge J, Murphy DN, Newman V, Nuhn M, Ogeh D, Ong CK, Parker A, Patricio M, Riat HS, Schuilenburg H, Sheppard D, Sparrow H, Taylor K, Thormann A, Vullo A, Walts B, Zadissa A, Frankish A, Hunt SE, Kostadima M, Langridge N, Martin FJ, Muffato M, Perry E, Ruffier M, Staines DM, Trevanion SJ, Aken BL, Cunningham F, Yates A, Flicek P. Ensembl 2018. Nucleic Acids Res. 2018;46(D1):D754–61.
Psifidi A, Banos G, Matika O, Desta TT, Bettridge J, Hume DA, Dessie T, Christley R, Wigley P, Hanotte O, Kaiser P. Genome-wide association studies of immune, disease and production traits in indigenous chicken ecotypes. Genet Sel Evol. 2016;48(1):74.
Chicken QTLdb. U. S. NAGRP Bioinformatics Coordination Program, USDA. 2018. https://www.animalgenome.org/cgi-bin/QTLdb/GG/index. Accessed 10 Nov 2018.
Gu X, Feng C, Ma L, Song C, Wang Y, Da Y, Li H, Chen K, Ye S, Ge C, Hu X, Li N. Genome-wide association study of body weight in chicken F2 resource population. PLoS One. 2011;6(7):e21872.
Guo J, Sun C, Qu L, Shen M, Dou T, Ma M, Wang K, Yang N. Genetic architecture of bone quality variation in layer chickens revealed by a genome-wide association study. Sci Rep. 2017;7:45317.
Shen M, Qu L, Ma M, Dou T, Lu J, Guo J, Hu Y, Yi G, Yuan J, Sun C, Wang K, Yang N. Genome-wide association studies for comb traits in chickens. PLoS One. 2016;11(7):e0159081.
Liao R, Zhang X, Chen Q, Wang Z, Wang Q, Yang C, Pan Y. Genome-wide association study reveals novel variants for growth and egg traits in Dongxiang blue-shelled and white Leghorn chickens. Anim Genet. 2016;47(5):588–96.
Fan QC, Wu PF, Dai GJ, Zhang GX, Zhang T, Xue Q, Shi HQ, Wang JY. Identification of 19 loci for reproductive traits in a local Chinese chicken by genome-wide study. Genet Mol Res. 2017;16(1):gmr16019431.
Turley P, Walters RK, Maghzian O, Okbay A, Lee JJ, Fontana MA, Nguyen-Viet TA, Wedow R, Zacher M, Furlotte NA, Magnusson P, Oskarsson S, Johannesson M, Visscher PM, Laibson D, Cesarini D, Neale BM, Benjamin DJ. 23andMe research team; social science genetic association consortium. Multi-trait analysis of genome-wide association summary statistics using MTAG. Nat Genet. 2018;50(2):229–37.
Lapa MA. Criteria for evaluation and selection of poultry in order to improve the food and biotechnological qualities of eggs. Diss Cand Biol Sci. St. Petersburg, Pushkin: RRIFAGB. 2015. 133 p.
Poggenpoel DG, Ferreira GF, Hayes JP, du Preez JJ. Response to long-term selection for egg production in laying hens. Br Poult Sci. 1996;37(4):743–56.
Maniatis T, Fritsch EF, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor: Cold Spring Harbor Laboratory; 1982. p. 6.4.
Niknafs S, Nejati-Javaremi A, Mehrabani-Yeganeh H, Fatemi SA. Estimation of genetic parameters for body weight and egg production traits in Mazandaran native chicken. Trop Anim Health Prod. 2012;44(7):1437–43.
RStudio Team. RStudio: integrated development for R. Boston: RStudio, Inc.; 2015. https://www.rstudio.com/. Accessed 10 Nov 2018
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, Maller J, Sklar P, de Bakker PIW, Daly MJ, Sham PC. PLINK: a toolset for whole-genome association and population-based linkage analysis. Am J Hum Genet. 2007;81(3):559–75.
Browning BL, Browning SR. Genotype imputation with millions of reference samples. Am J Hum Genet. 2016;98(1):116–26.
Kang HM, Sul JH, Service SK, Zaitlen NA, Kong SY, Freimer NB, Sabatti C, Eskin E. Variance component model to account for sample structure in genome-wide association studies. Nat Genet. 2010;42(4):348–54.
Gao X. Multiple testing corrections for imputed SNPs. Genet Epidemiol. 2011;35(3):154–8.
Hornik K. R FAQ: Frequently asked questions on R. Version 2017-10-04. In: Comprehensive R Archive Network. The R Project for Statistical Computing: The R Foundation; 2017. https://cran.r-project.org/doc/FAQ/R-FAQ.html. Accessed 10 Nov 2018.
Turner SD. qqman: an R package for visualizing GWAS results using Q-Q and manhattan plots. BioRxiv. 2014. https://doi.org/10.1101/005165.
Zaitlen N, Kraft P, Patterson N, Pasaniuc B, Bhatia G, Pollack S, Price AL. Using extended genealogy to estimate components of heritability for 23 quantitative and dichotomous traits. PLoS Genet. 2013;9(5):e1003520.
Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet. 2011;88(1):76–82.
Kim SA, Cho CS, Kim SR, Bull SB, Yoo YJ. A new haplotype block detection method for dense genome sequencing data based on interval graph modeling of clusters of highly correlated SNPs. Bioinformatics. 2018;34(3):388–97.
National Center for Biotechnology Information (NCBI). U. S. National Library of Medicine, Bethesda, MD. https://www.ncbi.nlm.nih.gov/. Accessed 10 Nov 2018.
Stelzer G, Rosen N, Plaschkes I, Zimmerman S, Twik M, Fishilevich S, Stein TI, Nudel R, Lieder I, Mazor Y, Kaplan S, Dahary D, Warshawsky D, Guan-Golan Y, Kohn A, Rappaport N, Safran M, Lancet D. The GeneCards suite: from gene data mining to disease genome sequence analyses. Curr Protoc Bioinformatics. 2016;54:1.30.1–1.30.33.
Acknowledgements
We acknowledge the RRIFAGB CUC GCRECB for providing chicken samples.
Funding
The research was part of the Project No. 16–16-04060 funded by the Russian Science Foundation. Except the financial support, the funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
Poultry genotypes used during the current study are available from the corresponding author upon a reasonable request. In addition, the list of proposed genes and their position that were found in this study are provided in the Table 2 in this manuscript.
Author information
Authors and Affiliations
Contributions
AAK contributed to the experiment design, sampling, genotyping, data analysis, and writing the main text of the manuscript. ESF took part in phenotypic data collection and evaluation. OIS, OVM and TAL contributed to designing population selection programme, sampling, genotyping and work revision. NVD produced and analysed the data, and contributed to the experiment design and manuscript writing. KVP and AIM participated in the experiment design, data analysis and manuscript writing. MNR and DKG contributed to discussion of the results and manuscript writing. All listed authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the Ethical & Animal care committee of the RRIFAGB — Branch of the L. K. Ernst Federal Science Centre for Animal Husbandry. Animals were handled with respect to the Russian Federal Law No. 498-FZ on Responsible Treatment of Animals and on Amendments to Certain Legislative Acts of the Russian Federation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Table S1. Basic information for SNP markers per chromosome after quality control. (DOCX 29 kb)
Additional file 2:
Description of the YEF and EM traits recording. (DOCX 13 kb)
Additional file 3:
Figure S1. Day old chicks with the white (A) and yellow (B) down colour. (JPG 586 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Kudinov, A.A., Dementieva, N.V., Mitrofanova, O.V. et al. Genome-wide association studies targeting the yield of extraembryonic fluid and production traits in Russian White chickens. BMC Genomics 20, 270 (2019). https://doi.org/10.1186/s12864-019-5605-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-019-5605-5