Abstract
Background
Juvenile hormones (JH) and ecdysteroids control postembryonic development in insects. They serve as valuable targets for pest management. Hence, understanding the molecular mechanisms of their action is of crucial importance. CREB-binding protein (CBP) is a universal transcriptional co-regulator. It controls the expression of several genes including those from hormone signaling pathways through co-activation of many transcription factors. However, the role of CBP during postembryonic development in insects is not well understood. Therefore, we have studied the role of CBP in postembryonic development in Tribolium, a model coleopteran insect.
Results
CBP is ubiquitously expressed in the red flour beetle, Tribolium castaneum. RNA interference (RNAi) mediated knockdown of CBP resulted in a decrease in JH induction of Kr-h1 gene expression in Tribolium larvae and led to a block in their development. Moreover, the injection of CBP double-stranded RNA (dsRNA) showed lethal phenotypes within 8 days of injection. RNA-seq and subsequent differential gene expression analysis identified CBP target genes in Tribolium. Knockdown of CBP caused a decrease in the expression of 1306 genes coding for transcription factors and other proteins associated with growth and development. Depletion of CBP impaired the expression of several JH response genes (e.g., Kr-h1, Hairy, early trypsin) and ecdysone response genes (EcR, E74, E75, and broad complex). Further, GO enrichment analyses of the downregulated genes showed enrichment in different functions including developmental processes, pigmentation, anatomical structure development, regulation of biological and cellular processes, etc.
Conclusion
These data suggest diverse but crucial roles for CBP during postembryonic development in the coleopteran model insect, Tribolium. It can serve as a target for RNAi mediated pest management of this stored product pest.
Similar content being viewed by others
Background
The endocrine and developmental pathways regulate insect development. Larval growth, prior to metamorphosis, consists of a number of successive instars that are punctuated by molts that are regulated by major hormones, ecdysteroids and Juvenile Hormones (JH). 20- hydroxyecdysone (20E) is the major functional ecdysteroid that regulates molting and metamorphosis [1]. 20E binds to ecdysteroid receptor (EcR) and Ultraspiracle (USP) in target tissues leading to an upregulation of ecdysteroid-inducible genes including E75, E74, E93 and BRC [2,3,4]. Interestingly, most of these 20E inducible genes encode transcription factors that further regulate expression of downstream genes.
Insect juvenile hormone (JH) also regulates many aspects of insect life including reproduction and development. Recently, a bHLH transcription factor methoprene-tolerant (Met) is identified as a JH receptor in the fruit fly, Drosophila melanogaster and other model insects including Bombyx mori, Aedes aegypti, Blattella germanica and Tribolium castaneum (see references [5, 6] for review). Further studies on JH signaling pathway revealed mechanisms of JH action as well as its cross-talk with 20-hydroxyecdysone (20E), insulin signaling and WNT pathways [7,8,9,10,11,12,13,14,15]. Hundreds of genes regulated by JH have been identified in these insects, and one gene consistently identified as an important player in JH action is krüppel homolog 1 (Kr-h1) [16,17,18,19,20,21]. The Kr-h1 expression is regulated by both JH and 20-hydroxyecdysone [22, 23]. The expression of Kr-h1 is directly induced by JH through Met, steroid receptor co-activator (SRC) and juvenile hormone response elements (JHRE) present in the promoter region [18, 24, 25].
Significant advances have been made in insect endocrinology research during the past few years. These studies have led to the discovery of major players including EcR, USP, E75, E73, E93, BRC, Met, Kr-h1 in 20E and JH signaling pathways [2,3,4, 6]. However, the regulatory mechanisms triggering such an orchestrated expression of different genes, transcription factors, coactivators, and repressors during the molting and metamorphosis in insects are not yet fully understood. Specifically, our knowledge regarding the molecular mechanisms underlying JH action is still limited.
CREB-binding protein (CBP) and its vertebrate paralog p300 are considered as one of the most promiscuous transcriptional co-regulator discovered to date with participation in the activities of hundreds of different transcription factors [26]. In addition to other properties, CBP and p300 have histone acetyltransferase (HAT) activity and act as hubs in transcription networking with more than 400 interaction partners, most of them are transcription factors and growth regulators [27, 28]. Thus, CBP is an important player in the development and may be involved in hormone signaling pathways in insects. In Drosophila melanogaster, the CBP homolog known as Nejire regulates embryonic segmental polarity during embryogenesis through Hedgehog and Wingless signaling pathways [29, 30] and dorsal-ventral patterning through the TGF-β signaling pathway [31].
During the postembryonic development in Drosophila, the contribution of Nejire to ecdysone signaling pathway has been reported. CBP was shown to play critical roles in initiating dendrite pruning. The HAT activity of CBP involved in acetylation of H3K27 is required for sox14 expression, which is an ecdysone response gene [32]. Acetylated H3K23 is localized to the promoters of Eip74EF and Eip75B, the 20E-induced transcription factors that play key roles in ecdysteroid action, and the acetylation levels of H3K23 correlate with the 20E-induced expression of these genes. Here also, acetylation is promoted by Nejire, the CBP homolog [33]. In Drosophila, lysine acetylation sites have been identified in the proteome using high-resolution mass spectrometry [34]. The sites of such modifications are highly conserved between humans and fruit flies. Furthermore, a study comparing lysine acetylation sites among fruit fly, human, nematode and zebrafish showed more conservation in the acetylated lysine residues than in the non-acetylated lysine residues [34].
However, there is limited information on the role of CBP or Nejire in postembryonic development of insects. Specifically, the function of CBP in JH action is not well studied. Recently, Fernandez et al., [35] showed that CBP plays a critical role in the regulation of metamorphosis in Blattella. In the current study, we employed RNAi and RNA sequencing to determine the function of CBP in the model insect, the red flour beetle, Tribolium.
Results
CBP expression in Tribolium larvae
To study the expression of CBP gene during larval development, whole larvae and pupae were collected for RNA extraction at 24 h intervals during penultimate and final instar larval and pupal stages. qRT-PCR was performed with gene specific primers (Additional file 1: Table S1). The CBP mRNA was detected in all the stages tested (Fig. 1). The CBP mRNA levels were lower during the penultimate instar and early stages of the final instar, then the mRNA levels gradually increased and reached the maximum levels at 48 h after metamorphosis into the pupal stage. In general, higher levels of CBP mRNA were detected during the pupal stage when compared to its levels during the larval stages (Fig. 1).
Relative CBP mRNA levels in Tribolium determined by qRT-PCR. Samples were collected at 24 h intervals during penultimate larvae, final instar larvae and pupal stages. Total RNA was extracted from pools of two larvae/pupae for each time period and converted to cDNA. The cDNA and gene-specific primers were used to quantify CBP mRNA levels using Ribosomal Protein 49 (Rp49) mRNA levels for normalization. The CBP mRNA levels in newly molted penultimate larvae were set as 1. Mean + SE of four replications were shown (Letters represent significance at 95% CI)
CBP RNAi affects growth and induces mortality
To understand the role of CBP in the regulation of larval and pupal development and metamorphosis, we employed RNAi mediated gene knockdown. The CBP double-stranded RNA (dsCBP) was injected into the newly molted last instar larvae, newly formed pupae, and newly emerged adults. Mortality and developmental defects were recorded. By about 4 days after the injection of dsCBP, the final instar larvae stopped feeding and growing, and remained smaller and turned dark brown color when compared to the control larvae injected with dsmalE (Fig. 2a). The CBP knockdown caused an arrest in development of the larvae during the quiescent stage prior to metamorphosis into pupal stage. The dsCBP injected larvae remained small and died after a few days. The dsCBP injected larvae also showed dark melanized areas in the midgut region of the alimentary canal (Fig. 2d). The dsmalE injected control larvae grew normally with no melanized areas in the midgut region of the alimentary canal and pupated after 5 days. Injection of dsCBP resulted in a significant decrease in CBP mRNA levels and caused 100% mortality (Fig. 2g&j). Injection of dsCBP into newly formed pupae blocked growth and development of pupae as well. The differentiation of compound eyes was impaired by depletion of CBP expression in newly formed pupae. Furthermore, pupal size was reduced in dsCBP-injected pupae, and the midgut region of the alimentary canal developed dark yellowish areas (Fig. 2b&e). The dsmalE-injected pupae emerged as normal adults in about 5 days after treatment. Injection of dsCBP into newly formed pupae resulted in a significant decrease in CBP mRNA levels and caused 100% mortality (Fig. 2h&k). Injection of dsCBP into newly emerged adults did not show any distinct phenotype after 4 days (Fig. 2c). Interestingly, the alimentary canal dissected from the CBP RNAi adults showed dark areas in the midgut region similar those observed in the larvae and pupae (Fig. 2f). Injection of dsCBP into newly formed adults resulted in a significant decrease in CBP mRNA levels and caused 100% mortality (Fig. 2i&l).
CBP knockdown causes lethality in larvae, pupae and adults of Tribolium. dsCBP was injected into newly molted last instar larvae, newly formed pupae and newly emerged adults. Phenotypes observed after 4 days after dsCBP injection into larvae (a), pupae (b) and adults (c) respectively. Dissected alimentary canals (mostly midgut region) from dsmalE and dsCBP injected larvae (d), pupae (e) and adults (f). Arrows point to the enlarged areas of the alimentary canals that showed darkened regions. Knockdown efficiency at 24 h after dsCBP injection in larvae (g), pupae (h) and adults (i). Percent mortality observed at 8 days after dsCBP injection into larvae (j), pupae (k) and adults (l). Scale Bar: 1 mm. [One way AVOVA, * P < 0.05]
CBP is required for expression of JH-response gene, Kr-h1
To determine whether CBP is required for JH-induced gene expression in the larvae, CBP was knockdown by injection of dsCBP into the newly ecdysed final instar larvae. Because the endogenous JH III levels are low in day 3 final instar larvae [36], the JH analog, hydroprene, was topically applied to these insects at 72 h after injection of dsCBP or control dsmalE. For control, cyclohexane was applied on dsmalE and dsCBP injected larvae. At 6 h after application of hydroprene or cyclohexane, the total RNA was isolated and utilized to determine relative mRNA levels using qRT-PCR. The CBP mRNA levels decreased by about 70% in dsCBP injected insects when compared to its levels in control insects injected with dsmalE (Fig. 3a). A significant reduction in Kr-h1 mRNA levels was detected in larvae injected with dsCBP when compared to its levels in control larvae injected with dsmalE after both cyclohexane and hydroprene treatments (Fig. 3b). These results showed that CBP is required for Kr-h1 expression in the larvae.
CBP is required for expression of Kr-h1 in Tribolium larvae. The newly emerged final instar larvae were injected with 1 μg of either dsmalE or dsCBP. Seventy-two hours later, 0.5 μl of 10 μM hydroprene or cyclohexane (control) was topically applied. Six hours after the application, total RNA was isolated and used to quantify mRNA levels of CBP (a) and Kr-h1 (b) in each treatment by qRT-PCR using RP49 as a control. The data shown are M + SE (n = 3). (Letters represent significance at 95% CI)
Differential gene expression (DGE) analysis in CBP knockdown larvae
To identify genes regulated by CBP, newly molted Tribolium last instar larvae were injected with dsmalE (control) or dsCBP, RNA isolated was from the larvae at 12 h after dsRNA injection and used for library preparation and sequencing. Illumina HiSeq4000 sequencing resulted in 147.9 million reads (i.e., 28.2, 31.7, 50.7, 37.2 million) for control samples and 109.6 million reads (i.e.,25.7, 22.3, 34.3, 27.2 million) for CBP knockdown samples with over 21.3 billion bp sequence information altogether and an estimated transcriptome coverage ranging from 23× to 52× for each of the four biological replicates. After quality control measures, 110.6 million reads (~94%) from control samples and 75.8 million reads (~88%) from CBP knockdown samples were mapped back to the reference genome of Tribolium (Table 1). After RPKM-based normalization of the gene expression, DGE was performed using the EDGE analysis tool, and the overall pattern of gene expression in control and CBP knockdown samples was represented as a heatmap (Fig. 4a). The expression differences within the replicates of treatment are smaller than between the treatments, thus, the replicates of each treatment cluster together (Fig. 4b). Differentially expressed genes are shown as a volcano plot with red dots indicating the statistically significant (p < 0.01 and ≥2-fold) expression differences between CBP (treatment) and malE (control) dsRNA-injected larvae (Fig. 4c). The expression levels of CBP in the larvae injected with dsCBP were >1.5-fold (EDGE test, p < 0.02) lower when compared to that of the control larvae. Approximately 1800 genes were differentially expressed, and of them, 1306 were significantly downregulated (p < 0.01, ≤2-fold) after CBP RNAi (Fig. 4d). Details on the Blast2GO hits in the NR databases, hit accessions, functional annotations, and the relative expression levels of these 1306 downregulated genes across the respective RNA seq samples can be found in Additional file 1: Figure S3 and Additional file 2: Excel file S1. Many of these downregulated genes have epifactor domains (Additional file 1: Figure S4) and belong to signaling pathways including FoxO, WNT, and mTOR (Additional file 1: Figure S5 and Supporting information S1). Also, GO enrichment analyses of 1306 downregulated genes revealed considerable enrichment in different molecular functions including oxidoreductase activity, hydrolase activity, transporter activity and molecular transducer activity. Biological processes such as developmental process, immune response, pigmentation, reproduction and regulation of cellular process were also enriched among the downregulated genes (Fig. 5). We identified 52 genes by K-mer cluster analysis that showed similar expression profile as Kr-h1, one of the important players in JH action (Fig. 6a, Additional file 3: Excel file S2). CBP RNAi also affected the expression of several genes with DNA-binding domains such as C2H2-type Zinc finger, BTB/PAZ, and bHLH that are known to play significant roles in insect development (Fig. 6b).
Gene expression analysis of RNA isolated from dsmalE (control, M1-M4) and dsCBP injected (C1-C4) last instar Tribolium larvae using the empirical analysis of DGE (EDGE) algorithm in CLC Genomics Workbench (Version: 9.5.9) and WEGO. a Heatmap showing the overall gene expression pattern of all four biological replicates from malE or CBP dsRNA injected larvae. The color spectrum, stretching from blue to red, represents TMM normalized expression values obtained after EDGE analysis. b Hierarchical clustering based on the overall gene expression pattern showing the relative differences within and among the biological replicates from malE or CBP dsRNA injected larvae. c Volcano plot of expression data after EDGE analysis. In the plot, log2 of fold change between the malE and CBP dsRNA-treated insects are plotted against −log10 (p), where p is a probability value (for a given gene) that is associated with the EDGE comparison of the two groups of samples. The red dots indicate the number of significantly up- and down-regulated genes using p < 0.01 and ±2-fold change as the cutoff threshold. d Heatmap illustrating the TMM normalized expression values of 1306 significantly downregulated genes (p < 0.01, ≤2-fold ↓) after CBP knockdown in Tribolium larvae. Color-coding is the same as in figure (a)
GO enrichment analysis. WEGO output for GO enrichment analysis with 1306 genes that were downregulated in Tribolium larvae after CBP knockdown. The plot shows percent and number of overrepresented GO terms compared to the reference genome (Tribolium genome). Asterisks indicated the statistically significant (p < 0.05) enrichment of important GO categories mainly under cellular component, molecular function and biological process categories after CBP RNAi
Post-EDGE data analysis. a Heatmap representing the expression levels of a subset of 53 genes in malE and CBP dsRNA-treated samples that clustered together with Kr-h1 after K-mer featured clustering of the data set containing 1306 downregulated genes in larvae injected with CBP dsRNA. b Heatmap showing the expression profile of Zinc finger, BTB/POZ, and HLH DNA-binding domain-containing genes in Tribolium larvae injected with CBP dsRNA. c Validation of RNA seq expression data by qRT-PCR using a subset of 20 genes. The fold change values obtained by qRT-PCR and RNA seq for each gene are plotted as a heatmap. Four biological replicates for each treatment (malE and CBP) were used for qRT-PCR analysis, and the mean fold change value of each gene was used in the heatmap. The experiment was repeated twice with comparable results
qRT-PCR validation
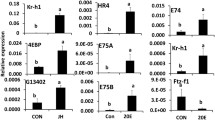
The data on the relative expression of the selected subset of 20 genes using qRT-PCR showed a positive correlation with the RNA seq data (Fig. 6c and Additional file 1: Figure S6). For example, a 5 and 13-fold decrease in Kr-h1 mRNA levels were detected in CBP knockdown larvae by qRT-PCR and RNA seq analyses respectively. The trends of gene expression are the same between the RNA seq and qRT-PCR analyses, but the magnitude of the change is different due to the varied sensitivity of the analysis platforms. The qRT-PCR did not detect any statistically significant change in the expression of TcMet, but RNA seq data predicted a 2.8-fold decrease (p < 0.01) in TcMet expression in CBP knockdown larvae. The details on the comparison of the expression levels of 20 selected genes by qRT-PCR and RNA seq are shown in Fig. 7.
Relative mRNA levels of a subset of 20 genes selected from the downregulated genes in dsCBP-injected larvae. Relative mRNA levels of these genes were determined by qRT-PCR as described in Fig. 1 legend. Each panel represents the relative mRNA levels of one target gene in dsmalE and dsCBP treated samples. Mean ± S.E (n = 4) are shown. [One-Way AVOVA, * P < 0.05, ** P < 0.01, *** P < 0.001]
Discussion
More than two decades have passed since the discovery of CBP as a transcriptional co-activator due to the presence multiple protein binding domains [37]. However, in insects, most of the studies on this transcriptional coregulator, CBP, are restricted to the model insect, Drosophila and its embryonic stage [29,30,31, 38,39,40,41]. There is limited information available about its impact on postembryonic development in insects. Recently, Fernandez-Nicolas et al., have reported on the contribution of CBP to the postembryonic development of Blattella. Our studies have extended the effort in the similar direction by examining the involvement of CBP in postembryonic development of a coleopteran model insect, Tribolium. CBP is ubiquitously expressed in the red flour beetle. The stage specific expression levels suggest that CBP is necessary during all stages, especially during quiescent and pupal stages (Fig. 1). Knockdown of CBP by RNAi is lethal during all beetle life stages tested, which indeed account for the fundamental role of CBP in Tribolium. Furthermore, interesting phenotypes have been observed in CBP RNAi larvae i.e., reduced growth and increase in melanization in the alimentary canal. The JH driven increase in the expression of Kr-h1, a major contributor to JH-dependent antimetamorphic action [19], has also decreased after injection of dsCBP indicating the plausible function of CBP as a coactivator in JH response gene expression (Fig. 3b).
Gene expression is a multi-layered process, regulated by many factors including histone and DNA modifiers, DNA-binding proteins, small RNAs, post-translational modifiers of nuclear proteins, and proteins involved in nuclear-cytoplasmic shuttling of transcription factors [24, 42,43,44,45]. Gene activation involves stripping of some or all of the silencing components from the promoter regions and recruiting proteins including activators and co-activators involved in transcription. Knockdown of CBP has caused significant downregulation of 1306 genes in Tribolium larvae. Many of these genes are associated with cellular and biological process regulation, pigmentation, developmental processes including anatomical structure development, and other key regulatory functions as revealed by GO enrichment analysis. Moreover, CBP knockdown also affected the cellular immune response, pigmentation, and metabolic process, which may have caused the enhanced melanization areas detected in the midgut of CBP knockdown animals (Fig. 2).
Depletion of CBP impairs hormone signaling
JH signaling
The presence of JH and expression of JH-response gene, Kr-h1, during larval development ensures molting whereas a drop in JH levels and expression of Kr-h1 induce metamorphosis in the presence of ecdysteroids. In Tribolium, Kr-h1 levels decrease at the end of the larval stage due to a decrease in JH biosynthesis, which leads to initiation of metamorphosis [19]. In this insect, application of JH analog, hydroprene extends larval stage and induce supernumerary larval molts [46] The Kr-h1 mRNA levels decreased by 13-fold in CBP knockdown larvae compared to that in the control larvae, further confirming a role for CBP in larval growth and metamorphosis. In D.melanogaster, the Kr-h1 mediates JH action during larval stages and 20E signaling during larval-pupal metamorphosis [23, 47,48,49]. Kr-h1 is a transcription factor containing a DNA-binding domain consisting of C2H2 zinc finger motifs and amino acid spacers connecting them [50]. Genes coding for seven additional C2H2 zinc finger domain-containing proteins including BRC are also downregulated in CBP knockdown larvae (Fig. 6b). The BRC proteins are coded by one of the early ecdysone response genes [51] that codes for a complex of proteins involved gene regulation during insect development and works as a key specifier of the pupal commitment [52, 53]. Interestingly, JH can also regulate BRC expression and is inducible by exogenous application of JH mimic during pupal stage [54,55,56]. Normally, BRC expression in Tribolium increases at the end of final instar larval stage following a reduction in Kr-h1 expression [54,55,56,57]. Another JH target gene, hairy, is also affected by CBP RNAi and the role of hairy as a target gene of Met in the JH gene repression hierarchy in Aedes aegypti has been reported recently by Saha et al., [58]. Moreover, early trypsin genes whose expression is also controlled by JH [59] are downregulated in CBP RNAi larvae. The expression of trypsin-1, trypsin-7 and trypsin II-P29 are reduced by 174, 128 and 29-fold respectively in CBP knockdown larvae when compared to their expression in control larvae (Additional file 2: Excel file S1). However, the expression of TcSRC is unaffected by CBP RNAi. TcSRC and TcMet co-regulate expression of several JH response genes in Tribolium [10]. The RNA seq analysis predicted downregulation of Met in CBP RNAi larvae. However, a decrease in TcMet expression in CBP RNAi larvae was not observed in the qRT-PCR experiment. TcMet is a JH receptor and contains a basic helix-loop-helix DNA-binding domain (bHLH DNA-binding domain). Genes coding for other bHLH DNA-binding domain-containing proteins including N-myc, AP-4, and tango, the aryl hydrocarbon receptor nuclear translocator homolog identified previously in Tribolium [60] are downregulated in CBP RNAi larvae. N-myc controls cell fate by regulating gene transcription, and the CBP was shown to act as a Myc co-activator and stimulates Myc-dependent transcription of gene expression in a HAT domain-dependent manner [61].
Ecdysone signaling
Depletion of CBP affected ecdysone-inducible genes such as ecdysone receptor (EcR), E74, E75, and BRC. EcR is one of the key players in ecdysone signaling cascade. 20E binds to its receptor EcR and induces the expression a series of early and early-late response genes that codes for additional transcription factors that control gene expression cascades [62]. The activity of EcR in dendrite pruning in Drosophila nerves is affected by CBP [32]. One possible explanation for these results is that the CBP indeed affects the activity of EcR similar to that in steroid receptors described in mammals [63]. The CBP may also interfere with the ecdysteroid biosynthesis as reported for steroid biosynthesis in vertebrates by modulating the expression of Cytochrome P450 enzymes involved in steroid hormone biosynthesis [64, 65]. The expression of genes coding for multiple Cytochrome p450 family members is also affected by CBP knockdown. One of them is CYP307B1, a member of the Halloween family that is involved in ecdysteroid biosynthesis [66]. A few other members of the CYP4 family (CYP4Q1, CYP4Q2, and CYP4Q7) are suppressed by CBP RNAi in Tribolium. The CYP4 group enzymes are known to be involved in the synthesis and metabolism of endogenous hormones and other compounds [67].
The E74 is induced directly by ecdysone and encodes transcription factors regulating ecdysone primary- and secondary-response genes [68]. E75 plays an important role in JH and ecdysone crosstalk and codes for four isoforms [69]. E75A is one of the isoforms with the complete DNA-binding domain, and CBP RNAi suppressed its expression. E75A, one of the transcriptional targets of JH, regulates the timing of metamorphosis in Drosophila by suppressing the expression of BRC during the early instars [70]. It is also expressed during each of the ecdysone pulses that trigger larval molting [71]. Moreover, E75A protein functions as a transcriptional repressor in vivo targeting ecdysone inducible gene, FTZ-F1 (Fushi tarazu binding factor 1), a nuclear hormone receptor, and a possible component of JH signaling pathway [71, 72]. Similar findings are documented by Fernandez-Nicolas et al., where CBP depletion affects the expression of E75A, E75B and HR3A genes from early ecdysone signaling cascade in Blattella. The observed delayed molting in CBP RNAi B. germanica larvae was predicted due to the reduced expression of above-mentioned genes. In the present study, the expression of HR3, HR4 and HR39 were reduced by 11, 8 and 3-fold after dsCBP injection (Additional file 2: Excel file S1).
Interestingly, the gene coding for Forkhead box O (FoxO) transcription factor was also downregulated in CBP dsRNA-treated larvae. In Bombyx mori, 20E induces FoxO expression and its nuclear localization that in turn upregulates expression of genes coding for lipases including brummer and acid lipase-1 for promoting lipolysis in the fat body cells during molting and pupation [73]. Recently, Lin et al., [74] showed that FoxO mediates the timing of pupation by regulating the ecdysteroid biosynthesis in the red flour beetle. Hence, it can be conceivable that CBP may regulate ecdysteroid biosynthesis by controlling the expression of FoxO. In Tribolium, vitellogenin (Vg) gene expression is under the control of JH and FoxO [8]. Vg is one of the downstream targets of bone morphogenesis protein (BMP) branch of a TGF-β signaling pathway that is involved in regulating the timing of metamorphic molt by influencing the ecdysteroid biosynthesis in Drosophila [75,76,77]. Depletion of CBP also reduces Vg gene expression as observed in cockroach. Moreover, the GO enrichment analysis also suggested an effect of CBP knockdown on reproduction (Fig. 5).
Impact of CBP RNAi on expression of genes involved in development
Insect growth and metamorphosis are regulated by the nutrient supply to the insect. For instance, tachykinin like peptide contributes to maintaining adequate food consumption during vitellogenesis, a period of high energy demand [78]. In the current study, tachykinin like peptide receptor 86C was downregulated (ca. 40-fold) in CBP RNAi larvae. A similar experiment in cockroaches showed less food intake and slow growth due to a reduction in tachykinin and sulfakinin expression in CBP RNAi insects. In the present study, we did not measure the effect of CBP RNAi on food intake in Tribolium larvae, but the reduced size of the CBP RNAi larvae suggests reduced growth.
Furthermore, using K-mer clustering, we have identified 52 out of 1306 down-regulated genes with similar expression profile as Kr-h1. Some of these 52 genes are known to play a central role in insect growth. These genes include Chitinase 10 (TcCHT10), hexamerin, apolipophorins, and fatty acid synthase. TcCHT10 contains multiple catalytic domains and plays a key role in larval molting, pupation, and adult metamorphosis [79]. Involvement of hexamerins in JH-dependent gene expression has been reported in a social insect, termite [80]. Apolipophorins serve as lipid transporters; however, Apolipophorin III, downregulated by CBP knockdown, is also known to play an important role in insect immunity [81, 82]. It is also involved in regulation of phenoloxidase system. Thus, suppression of Apolipophorin expression may upset the regulation of phenoloxidase system in Tribolium larvae resulting in a higher degree of melanization after the CBP knockdown (Fig. 2a, d). The present study also revealed that fatty acid synthase (FAS) expression was also regulated by TcCBP. The FAS is another important player during diapause preparation in insects and lipid synthesis [83]. The transcription of FAS is suppressed by JH and the JH receptor Met. The CBP RNAi also suppressed the expression of matrix metalloproteinase 2 (Mmp2), a protein involved in pre-pupal remodeling of the larval fat body [84].
Interestingly, genes coding for Cathepsin L, juvenile hormone esterase (JHE), solute carrier family member protein, juvenile acid O methyl transferase (JHAMT), cytochrome p450 (TC002552) that are induced by JH through Met [10], are also downregulated after CBP knockdown signifying the role of TcCBP in the expression of genes that require Met for their JH-mediated regulation.
Does CBP function as an epigenetic modulator in endocrine signaling?
In Drosophila, CBP functions as a histone acetyltransferase (HAT) in the 20E regulation of dendrite pruning through acetylation of H3K27; CBP also physically interacts with EcR, USP, and Brahma [32]. Histone acetyltransferase activity is also involved in the 20E–induced expression of Eip74EF and Eip75B, and CBP-mediated acetylation of histone H3K23 is required for this action [33]. Our present study does not monitor the impact of CBP-mediated acetylation of histones on the expression of genes related to Tribolium growth and metamorphosis. However, genes with epi-factor domains such as SET, PHD, Jmjc, LRR, C5HC2 zing finger, and Arid are downregulated after CBP knockdown (Additional file 1: Figure S4). These domains are known to be present in epigenetic enzymes such as histone lysine methylases (HMTs), histone demethylases (HDMs), lysine metyltransferases (KMTs), and lysine demethylases (KDMs) and play important roles in hormone signaling [85]. Genes containing bromodomain (BRD) and chromodomain (CHD) that recognize acetylated histones and methylated histones respectively are also affected by CBP RNAi. Hence, it is rather clear from our study that CBP may act as an epigenetic modulator by regulating expression of many epi-factor domain-containing proteins. Such epi-factor domain-containing proteins may regulate (i.e., epigenetic regulation) the expression of many genes associated with postembryonic development in Tribolium. However, detailed insights into the epigenetic regulation of hormone signaling by CBP requires studies using ChiP seq based or acetylome approaches in CBP RNAi larvae. These studies are currently underway in our laboratory.
Conclusions
CBP is expressed in all life stages of the red flour beetle. CBP RNAi causes lethal phenotypes in the larvae, which are indeed a cumulative consequence of reduced expression of many vital genes in CBP RNAi larvae. Moreover, CBP regulates the expression of a cascade of early and late response genes in JH and 20E signaling pathways by regulating expression of genes involved in the action of these hormones. Hence, CBP plays a diverse yet decisive role in the regulation of the postembryonic development in the coleopteran model insect, Tribolium.
Methods
Insect rearing
The wild-type GA-1 strain of Tribolium [86] were maintained on organic wheat flour with 10% yeast at 30 °C and 65 ± 5% RH inside insect rearing chambers as described by earlier co-workers [87]. The larvae and pupae were staged based on the size, color, and number of days after hatching. The final instar larvae were identified based on the untanned white cuticle, observed immediately after molting. White colored pupae were considered as newly molted pupae.
DsRNA injection and topical application of JH analog hydroprene
The newly emerged adults (within 6 h after emergence) or final instar larvae were anesthetized with ether vapor for 4–5 min and lined on a glass slide covered with 2-sided tape. The dsRNA was injected into the dorsal side of the first or second abdominal segment using an injection needle pulled from a glass capillary tube using a needle puller (Idaho Technology, Salt Lake City, UT). About 0.8–1 μg (0.1 μl) dsRNA was injected into each new male adult, pupae or each larva. The malE dsRNA prepared using a fragment of E. coli malE gene amplified using T7 primer (TAATACGACTCACT ATAGGG) and Litmus28iMal plasmid (New England Bio labs, Ipswich, MA) as a template was used as a control. The injected beetles were removed from the slide and reared on whole-wheat flour and 10% Baker’s yeast at 30 ± 1 °C for 72 h. For hormone treatment, hydroprene (1 μl of 10 μg/μl) was topically applied to adults and larvae after 72 h exposure to dsRNA. The animals were collected at 6 h after hydroprene application.
Total RNA was isolated using the TRI reagent (Molecular Research Center Inc., Cincinnati, OH). The DNA was eliminated from the total RNA using DNase I (Ambion Inc., Austin, TX) and 1 μg of total RNA for each sample was used for cDNA synthesis. cDNAs were used as templates to amplify fragments of target genes. The PCR products were used for dsRNA synthesis. The primers used in these experiments are listed in Additional file 1: Table S1. The MEGA script RNAi kit (Ambion Inc., Austin, TX) was used for dsRNA synthesis as described previously [10].
CBP knockdown, RNA seq library preparation, and sequencing
Final instar larvae were injected with 1 μg of malE or CBP dsRNA and maintained on organic wheat flour containing 10% yeast for 12, 24, 48 h. Larvae (four biological replicates) from each time point were collected and the total RNA was extracted from the whole larvae using the TRI reagent (Molecular Research Center Inc., Cincinnati, OH) and cDNA was synthesized using M-MLV reverse transcriptase (Thermo Fisher Scientific Inc., Waltham, MA). qRT-PCR was performed to evaluate the knockdown efficiency and its impact on JH signaling pathway using Kr-h1 gene expression as a molecular marker. Samples from CBP knockdown larvae that showed a decrease in Kr-h1 expression (12 h after injection of dsRNA, Additional file 1: Figure S1 A) were selected for RNA seq library preparation and sequencing. RNA seq libraries were prepared using 2 μg RNA per replicate per library following “Method B2” as described by Ma et al. [88]. The libraries were size selected (Additional file 1: Figure S1 B), multiplexed and sequenced on an Illumina Hi-seq 4000 sequencer (Sequencing and Genomics Technologies Center of Duke University, NC, USA). Raw sequencing data statistics are shown in Table 1.
Mapping, annotation, and DGE analysis
Raw reads after quality control (demultiplexing, trimming, adaptor removal) were mapped back to the published reference genome of GA-2 Tribolium strain using pre-optimized parameters (i.e. unique exon mapping, mismatch cost = 2, insertion cost = 3, deletion cost = 3, length fraction = 0.8, similarity fraction = 0.8) in CLC Genomic Workbench (Version 9.5.9, Qiagen Bioinformatics, Valencia, CA). After counting of the mapped reads, gene expression level was normalized for different dsRNA treatments (Additional file 1: Figure S2). Only uniquely mapped exon reads were considered for downstream analysis. Differential gene expression analysis was performed using the inbuilt CLC Genomic Workbench software tool, “Empirical analysis of DGE” (EDGE) employing standard parameters. A stringent p-value cutoff <0.01 was employed along with a ≥2-fold change to identify the differentially expressed genes between the malE and CBP dsRNA-treated samples. Using inbuilt K-mer clustering tool of CLC genomic workbench, differentially expressed genes were grouped into few clusters based on their expression features. Genes of interest were functionally annotated using the Blast2Go Pro plugin available with the CLC Genomic Workbench and represented using Web Gene Ontology Annotation Plot (WEGO) [89]. GO enrichment analysis (level 2) was performed by plotting the GO information of the target samples against all the GOs from Tribolium genome in WEGO. Domain search was carried out by searching the translated protein sequences of target genes against the Pfam domain database downloaded in CLC workbench. Furthermore, pathway analysis was conducted using the KEGG pathway database resources [90]. To validate the results of RNA seq data, expression of 20 selected genes was quantified using qRT-PCR. The selection of the genes was based on their potential function (i.e., DNA binding, transcription factor activity) and plausible impact on the JH signaling pathway and insect development. During qRT-PCR, a melt curve was generated to ensure single product amplification after each run. The expression levels of the target genes were calculated using 2 –ΔCt method, with RP49 (a ribosomal protein) serving as a reference housekeeping gene. Mean 2 –ΔCt values for each gene from malE and CBP knockdown group were used for fold change calculations.
Change history
06 August 2018
Following the publication of this article [1], the authors found that the primers listed for CREB-binding protein were not correct. This mistake occurred during assembly of the primer table and the authors apologize for this error. This correction does not change the data included in the paper, their interpretation nor the conclusions drawn.
Abbreviations
- 20E:
-
20- hydroxyecdysone
- BMP:
-
Bone morphogenesis protein
- BRD:
-
Bromodomain
- CBP:
-
CREB-binding protein
- CHD:
-
Chromodomain
- DGE:
-
Differential gene expression
- dsRNA:
-
double-stranded RNA
- EcR:
-
Ecdysteroid receptor
- FoxO:
-
Forkhead box O transcription factor
- FTZ-F1:
-
Fushi tarazu binding factor 1
- HAT:
-
Histone acetyltransferase
- HDM:
-
Histone demethylases
- JH:
-
Juvenile hormone
- JHRE:
-
Juvenile hormone response element
- KDM:
-
Lysine demethylases
- KMT:
-
Lysine metyltransferases
- Kr-h1:
-
Krüppel homolog 1
- Met:
-
Methoprene tolerant
- qRT-PCR:
-
quantitative reverse-transcriptase PCR
- SRC:
-
Steroid receptor co-activator
- USP:
-
Ultraspiracle
- Vg:
-
Vitellogenin
References
Nijhout HF, Callier V. Developmental mechanisms of body size and wing-body scaling in insects. Annu Rev Entomol. 2015;60:141–56.
Hall BL, Thummel CS. The RXR homolog ultraspiracle is an essential component of the Drosophila ecdysone receptor. Development. 1998;125(23):4709–17.
Huet F, Ruiz C, Richards G. Puffs and PCR: the in vivo dynamics of early gene expression during ecdysone responses in Drosophila. Development. 1993;118(2):613–27.
Yao T-P, Forman BM, Jiang Z, Cherbas L, Chen J-D, McKeown M, Cherbas P, Evans RM. Functional ecdysone receptor is the product of EcR and Ultraspiracle genes. Nature. 1993;366(6454):476–9.
Jindra M, Palli SR, Riddiford LM. The juvenile hormone signaling pathway in insect development. Annu Rev Entomol. 2013;58:181–204.
Jindra M, Belles X, Shinoda T. Molecular basis of juvenile hormone signaling. Curr Opin Insect Sci. 2015;11:39–46.
Mirth CK, Tang HY, Makohon-Moore SC, Salhadar S, Gokhale RH, Warner RD, Koyama T, Riddiford LM, Shingleton AW. Juvenile hormone regulates body size and perturbs insulin signaling in Drosophila. Proc Natl Acad Sci U S A. 2014;111(19):7018–23.
Sheng Z, Xu J, Bai H, Zhu F, Palli SR. Juvenile hormone regulates vitellogenin gene expression through insulin-like peptide signaling pathway in the red flour beetle, Tribolium castaneum. J Biol Chem. 2011;286(49):41924–36.
Abdou M, Peng C, Huang J, Zyaan O, Wang S, Li S, Wang J. Wnt signaling cross-talks with JH signaling by suppressing met and gce expression. PLoS One. 2011;6(11):e26772.
Zhang Z, Xu J, Sheng Z, Sui Y, Palli SR. Steroid receptor co-activator is required for juvenile hormone signal transduction through a bHLH-PAS transcription factor, methoprene tolerant. J Biol Chem. 2011;286(10):8437–47.
Charles JP, Iwema T, Epa VC, Takaki K, Rynes J, Jindra M. Ligand-binding properties of a juvenile hormone receptor, Methoprene-tolerant. Proc Natl Acad Sci U S A. 2011;108(52):21128–33.
Li M, Mead EA, Zhu J. Heterodimer of two bHLH-PAS proteins mediates juvenile hormone-induced gene expression. Proc Natl Acad Sci U S A. 2011;108(2):638–43.
Jindra M, Uhlirova M, Charles JP, Smykal V, Hill RJ. Genetic evidence for function of the bHLH-PAS protein Gce/met as a juvenile hormone receptor. PLoS Genet. 2015;11(7):e1005394.
Lozano J, Kayukawa T, Shinoda T, Belles X A. Role for Taiman in Insect Metamorphosis. PLoS Genet. 2014;10(10):e1004769.
Belles X, Santos CG. The MEKRE93 (Methoprene tolerant-Kruppel homolog 1-E93) pathway in the regulation of insect metamorphosis, and the homology of the pupal stage. Insect Biochem Mol Biol. 2014;52:60–8.
Minakuchi C, Zhou X, Riddiford LM. Kruppel homolog 1 (Kr-h1) mediates juvenile hormone action during metamorphosis of Drosophila melanogaster. Mech Dev. 2008;125(1–2):91–105.
Shin SW, Zou Z, Saha TT, Raikhel AS. bHLH-PAS heterodimer of methoprene-tolerant and cycle mediates circadian expression of juvenile hormone-induced mosquito genes. Proc Natl Acad Sci U S A. 2012;109(41):16576–81.
Kayukawa T, Minakuchi C, Namiki T, Togawa T, Yoshiyama M, Kamimura M, Mita K, Imanishi S, Kiuchi M, Ishikawa Y, et al. Transcriptional regulation of juvenile hormone-mediated induction of Kruppel homolog 1, a repressor of insect metamorphosis. Proc Natl Acad Sci U S A. 2012;109(29):11729–34.
Minakuchi C, Namiki T, Shinoda T. Kruppel homolog 1, an early juvenile hormone-response gene downstream of Methoprene-tolerant, mediates its anti-metamorphic action in the red flour beetle Tribolium castaneum. Dev Biol. 2009;325(2):341–50.
Lozano J, Belles X. Conserved repressive function of Kruppel homolog 1 on insect metamorphosis in hemimetabolous and holometabolous species. Sci Rep. 2011;1:163.
Zou Z, Saha TT, Roy S, Shin SW, Backman TWH, Girke T, White KP, Raikhel AS. Juvenile hormone and its receptor, methoprene-tolerant, control the dynamics of mosquito gene expression. Proc Natl Acad Sci U S A. 2013;110(24):E2173–81.
Benevolenskaya EV, Frolov MV, Birchler JA. Kruppel homolog (Kr h) is a dosage-dependent modifier of gene expression in Drosophila. Genet Res. 2000;75(2):137–42.
Pecasse F, Beck Y, Ruiz C, Richards G. Kruppel-homolog, a stage-specific modulator of the prepupal ecdysone response, is essential for Drosophila metamorphosis. Dev Biol. 2000;221(1):53–67.
Cui Y, Sui Y, Xu J, Zhu F, Palli SR. Juvenile hormone regulates Aedes aegypti Kruppel homolog 1 through a conserved E box motif. Insect Biochem Mol Biol. 2014;52:23–32.
Kayukawa T, Tateishi K, Shinoda T. Establishment of a versatile cell line for juvenile hormone signaling analysis in Tribolium castaneum. Sci Rep. 2013;3:1570.
Vo N, Goodman RH. CREB-binding protein and p300 in transcriptional regulation. J Biol Chem. 2001;276(17):13505–8.
McManus KJ, Hendzel MJ. CBP, a transcriptional coactivator and acetyltransferase. Biochem Cell Biol. 2001;79(3):253–66.
Janknecht R, Wells NJ, Hunter T. TGF-β-stimulated cooperation of Smad proteins with the coactivators CBP/p300. Genes Dev. 1998;12(14):2114–9.
Goodman RH, Smolik S. CBP/p300 in cell growth, transformation, and development. Genes Dev. 2000;14(13):1553–77.
Akimaru H, Chen Y, Dai P, Hou D-X, Nonaka M, Smolik SM, Armstrong S, Goodman RH, Ishii S. Drosophila CBP is a co-activator of cubitus interruptus in hedgehog signalling. Nature. 1997;386(6626):735.
Heldin C-H, Moustakas A. Role of Smads in TGFβ signaling. Cell Tissue Res. 2012;347(1):21–36.
Kirilly D, Wong JJ, Lim EK, Wang Y, Zhang H, Wang C, Liao Q, Wang H, Liou YC, Yu F. Intrinsic epigenetic factors cooperate with the steroid hormone ecdysone to govern dendrite pruning in drosophila. Neuron. 2011;72(1):86–100.
Bodai L, Zsindely N, Gaspar R, Kristo I, Komonyi O, Boros IM. Ecdysone induced gene expression is associated with acetylation of histone H3 lysine 23 in Drosophila melanogaster. PLoS One. 2012;7(7):e40565.
Weinert BT, Wagner SA, Horn H, Henriksen P, Liu WR, Olsen JV, Jensen LJ, Choudhary C. Proteome-wide mapping of the Drosophila acetylome demonstrates a high degree of conservation of lysine acetylation. Sci Signal. 2011;4(183):ra48.
Fernandez-Nicolas A, Belles X. CREB-binding protein contributes to the regulation of endocrine and developmental pathways in insect hemimetabolan pre-metamorphosis. Biochim Biophys Acta. 2016;1860(3):508–15.
Parthasarathy R, Sun Z, Bai H, Palli SR. Juvenile hormone regulation of vitellogenin synthesis in the red flour beetle, Tribolium castaneum. Insect Biochem Mol Biol. 2010;40(5):405–14.
Chrivia JC, Kwok RP, Lamb N, Hagiwara M, Montminy MR, Goodman RH. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365(6449):855–9.
Akimaru H, Hou D-X, Ishii S. Drosophila CBP is required for dorsal-dependent twist. Nat Genet. 1997;17:211.
Waltzer L, Bienz M. A function of CBP as a transcriptional co-activator during Dpp signalling. EMBO J. 1999;18(6):1630–41.
Holmqvist P-H, Boija A, Philip P, Crona F, Stenberg P, Mannervik M. Preferential genome targeting of the CBP co-activator by Rel and Smad proteins in early Drosophila melanogaster embryos. PLoS Genet. 2012;8(6):e1002769.
Peterson AJ, O’Connor MB. Strategies for exploring TGF-β signaling in Drosophila. Methods. 2014;68(1):183–93.
Tropberger P, Pott S, Keller C, Kamieniarz-Gdula K, Caron M, Richter F, Li G, Mittler G, Liu ET, Buhler M, et al. Regulation of transcription through acetylation of H3K122 on the lateral surface of the histone octamer. Cell. 2013;152(4):859–72.
Zeng J, Yi SV. DNA methylation and genome evolution in honeybee: gene length, expression, functional enrichment covary with the evolutionary signature of DNA methylation. Genome Biol Evol. 2010;2:770–80.
Zheng WW, Dong XM, Yin RH, Xu FF, Ning HM, Zhang MJ, Xu CW, Yang Y, Ding YL, Wang ZD, et al. EDAG positively regulates erythroid differentiation and modifies GATA1 acetylation through recruiting p300. Stem Cells. 2014;32(8):2278–89.
Varghese J, Cohen SM. microRNA miR-14 acts to modulate a positive autoregulatory loop controlling steroid hormone signaling in drosophila. Genes Dev. 2007;21(18):2277–82.
Parthasarathy R, Palli SR. Molecular analysis of juvenile hormone analogaction in controlling the metamorphosis of the redflour beetle, Tribolium castaneum. Arch Insect Biochem. 2009;70:57–70.
Minakuchi C, Namiki T, Yoshiyama M, Shinoda T. RNAi-mediated knockdown of juvenile hormone acid O-methyltransferase gene causes precocious metamorphosis in the red flour beetle Tribolium castaneum. FEBS J. 2008;275(11):2919–31.
Beckstead RB, Lam G, Thummel CS. The genomic response to 20-hydroxyecdysone at the onset of Drosophila metamorphosis. Genome Biol. 2005;6(12):R99.
Kang P, Chang K, Liu Y, Bouska M, Birnbaum A, Karashchuk G, Thakore R, Zheng W, Post S, Brent CS, Li S, Tatar M, Bai H. Drosophila Kruppel homolog 1 represses lipolysis through interaction with dFOXO. Sci Rep. 2017;7(1):16369.
Schuh R, Aicher W, Gaul U, Côte S, Preiss A, Maier D, Seifert E, Nauber U, Schröder C, Kemler R. A conserved family of nuclear proteins containing structural elements of the finger protein encoded by Krüppel, a drosophila segmentation gene. Cell. 1986;47(6):1025–32.
Bayer CA, Holley B, Fristrom JW. A switch inBroad-ComplexZinc-finger isoform expression is regulated posttranscriptionally during the metamorphosis of Drosophila imaginal discs. Dev Biol. 1996;177(1):1–14.
Karim FD, Guild GM, Thummel CS. The Drosophila broad-complex plays a key role in controlling ecdysone-regulated gene expression at the onset of metamorphosis. Development. 1993;118(3):977–88.
Zhou B, Riddiford LM. Hormonal regulation and patterning of the broad-complex in the epidermis and wing discs of the tobacco hornworm, Manduca sexta. Dev Biol. 2001;231(1):125–37.
Konopova B, Jindra M. Broad-complex acts downstream of met in juvenile hormone signaling to coordinate primitive holometabolan metamorphosis. Development. 2008;135(3):559–68.
Parthasarathy R, Tan A, Bai H, Palli SR. Transcription factor broad suppresses precocious development of adult structures during larval-pupal metamorphosis in the red flour beetle, Tribolium castaneum. Mech Dev. 2008;125(3–4):299–313.
Suzuki Y, Truman JW, Riddiford LM. The role of broad in the development of Tribolium castaneum: implications for the evolution of the holometabolous insect pupa. Development. 2008;135(3):569–77.
Thummel CS. The Drosophila E74 promoter contains essential sequences downstream from the start site of transcription. Genes Dev. 1989;3(6):782–92.
Saha TT, Shin SW, Dou W, Roy S, Zhao B, Hou Y, Wang XL, Zou Z, Girke T, Raikhel AS. Hairy and Groucho mediate the action of juvenile hormone receptor Methoprene-tolerant in gene repression. Proc Natl Acad Sci U S A. 2016;113(6):E735–43.
Noriega F, Shah D, Wells M. Juvenile hormone controls early trypsin gene transcription in the midgut ofAedes aegypti. Insect Mol Biol. 1997;6(1):63–6.
Bitra K, Palli SR. The members of bHLH transcription factor superfamily are required for female reproduction in the red flour beetle, Tribolium castaneum. J Insect Physiol. 2010;56(10):1481–9.
Faiola F, Liu X, Lo S, Pan S, Zhang K, Lymar E, Farina A, Martinez E. Dual regulation of c-Myc by p300 via acetylation-dependent control of Myc protein turnover and coactivation of Myc-induced transcription. Mol Cell Biol. 2005;25(23):10220–34.
King-Jones K, Thummel CS. Nuclear receptors--a perspective from Drosophila. Nat Rev Genet. 2005;6(4):311.
Kino T, Nordeen SK, Chrousos GP. Conditional modulation of glucocorticoid receptor activities by CREB-binding protein (CBP) and p300. J Steroid Biochem Mol Biol. 1999;70(1):15–25.
Gizard F, Lavallée B, DeWitte F, Hum DW. A novel zinc finger protein TReP-132 interacts with CBP/p300 to regulate human CYP11A1 gene expression. J Biol Chem. 2001;276(36):33881–92.
Monté D, DeWitte F, Hum DW. Regulation of the human P450scc gene by steroidogenic factor 1 is mediated by CBP/p300. J Biol Chem. 1998;273(8):4585–91.
Namiki T, Niwa R, Sakudoh T, Shirai K-I, Takeuchi H, Kataoka H. Cytochrome P450 CYP307A1/spook: a regulator for ecdysone synthesis in insects. Biochem Bioph Res Co. 2005;337(1):367–74.
Sutherland T, Unnithan G, Andersen J, Evans P, Murataliev M, Szabo L, Mash E, Bowers W, Feyereisen R. A cytochrome P450 terpenoid hydroxylase linked to the suppression of insect juvenile hormone synthesis. Proc Natl Acad Sci U S A. 1998;95(22):12884–9.
Thummel CS, Burtis KC, Hogness DS. Spatial and temporal patterns of E74 transcription during Drosophila development. Cell. 1990;61(1):101–11.
Segraves WA, Hogness DS. The E75 ecdysone-inducible gene responsible for the 75B early puff in drosophila encodes two new members of the steroid receptor superfamily. Genes Dev. 1990;4(2):204–19.
Dubrovsky EB, Dubrovskaya VA, Berger EM. Hormonal regulation and functional role of Drosophila E75A orphan nuclear receptor in the juvenile hormone signaling pathway. Dev Biol. 2004;268(2):258–70.
Cáceres L, Necakov AS, Schwartz C, Kimber S, Roberts IJ, Krause HM. Nitric oxide coordinates metabolism, growth, and development via the nuclear receptor E75. Genes Dev. 2011;25(14):1476–85.
Dubrovsky EB, Dubrovskaya VA, Bernardo T, Otte V, DiFilippo R, Bryan H. The drosophila FTZ-F1 nuclear receptor mediates juvenile hormone activation of E75A gene expression through an intracellular pathway. J Biol Chem. 2011;286(38):33689–700.
Hossain MS, Liu Y, Zhou S, Li K, Tian L, Li S. 20-Hydroxyecdysone-induced transcriptional activity of FoxO upregulates brummer and acid lipase-1 and promotes lipolysis in Bombyx fat body. Insect Biochem Mol Biol. 2013;43(9):829–38.
Lin X, Yu N, Smagghe G. FoxO mediates the timing of pupation through regulating ecdysteroid biosynthesis in the red flour beetle, Tribolium castaneum. Gen Comp Endocr. 2017.
Kim J, Sebring A, Esch JJ, Kraus ME, Vorwerk K, Magee J, Carroll SB. Integration of positional signals and regulation of wing formation and identity by Drosophila vestigial gene. Nature. 1996;382(6587):133–8.
Brummel T, Abdollah S, Haerry TE, Shimell MJ, Merriam J, Raftery L, Wrana JL, O’Connor MB. The drosophila activin receptor baboon signals through dSmad2 and controls cell proliferation but not patterning during larval development. Genes Dev. 1999;13(1):98–111.
Gibbens YY, Warren JT, Gilbert LI, O’Connor MB. Neuroendocrine regulation of drosophila metamorphosis requires TGFβ/Activin signaling. Development. 2011;138(13):2693–703.
Pascual N, Maestro JL, Chiva C, Andreu D, Bellés X. Identification of a tachykinin-related peptide with orexigenic properties in the German cockroach. Peptides. 2008;29(3):386–92.
Zhu Q, Arakane Y, Beeman RW, Kramer KJ, Muthukrishnan S. Functional specialization among insect chitinase family genes revealed by RNA interference. Proc Natl Acad Sci U S A. 2008;105(18):6650–5.
Zhou X, Tarver MR, Scharf ME. Hexamerin-based regulation of juvenile hormone-dependent gene expression underlies phenotypic plasticity in a social insect. Development. 2007;134(3):601–10.
Zdybicka-Barabas A, Cytryńska M. Apolipophorins and insect immune response. ISJ. 2013;10:58–68.
Patnaik BB, Patnaik HH, Park KB, Jo YH, Lee YS, Han YS. Silencing of apolipophorin-III causes abnormal adult morphological phenotype and susceptibility to Listeria monocytogenes infection in Tenebrio molitor. Entomol Res. 2015;45(2):116–21.
Tan Q-Q, Liu W, Zhu F, Lei C-L, Wang X-P. Fatty acid synthase 2 contributes to diapause preparation in a beetle by regulating lipid accumulation and stress tolerance genes expression. Sci Rep. 2017;7:40509.
Bond ND, Nelliot A, Bernardo MK, Ayerh MA, Gorski KA, Hoshizaki DK, Woodard CT. ssFTZ-F1 and matrix metalloproteinase 2 are required for fat-body remodeling in drosophila. Dev Biol. 2011;360(2):286–96.
Huang Y, Dennehey B, Shi X. Histone lysine methylation, demethylation, and hormonal gene regulation. In: Mandal SS, editor. Gene regulation, epigenetics and hormone signaling: Weiheim: Wiley-VCH Verlag GmbH & Co. KGaA; 2017. p. 59–100.
Haliscak JP, Beeman RW. Status of malathion resistance in five genera of beetles infesting farm-stored corn, wheat, and oats in the United States. J Econ Entomol. 1983;76(4):717–22.
Parthasarathy R, Tan A, Sun Z, Chen Z, Rankin M, Palli S. Juvenile hormone regulation of male accessory gland activity in the red flour beetle, Tribolium castaneum. Mech Dev. 2009;126(7):563–79.
Ma L, Pati PK, Liu M, Li QQ, Hunt AG. High throughput characterizations of poly (a) site choice in plants. Methods. 2014;67(1):74–83.
Ye J, Fang L, Zheng H, Zhang Y, Chen J, Zhang Z, Wang J, Li S, Li R, Bolund L. WEGO: a web tool for plotting GO annotations. Nucleic Acids Res. 2006;34(suppl 2):W293–7.
Kanehisa M, Goto S, Sato Y, Kawashima M, Furumichi M, Tanabe M. Data, information, knowledge and principle: back to metabolism in KEGG. Nucleic Acids Res. 2014;42(D1):D199–205.
Acknowledgments
This is publication number 17-08-118 from the KentuckyAgricultural Experimental Station and published with the approval of the director.
Funding
This work is supported by a grant from the National Institutes of Health (GM070559-11) and the National Institute of Food and Agriculture, USDA, HATCH under 2351177000. The funding bodies played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
We have deposited the short read (Illumina HiSeq4000) data with the following accession numbers, Study, PRJNA383401 and SRP104247; Samples, SRS2131977-SRS2131984); Experiment, SRX2745604-SRX2745604- SRX2745610 and Run, SRR5457553- SRR5457559.
Author information
Authors and Affiliations
Contributions
AR and SRP designed research, AR and SG conducted the experiments, AR and SRP wrote the manuscript, and all authors approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
No field permissions were required. The insects used in these studies were reared in our laboratory since 2006 from a starter culture received from USDA-ARS.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1: Table S1.
Sequences of primers used in the experiments. Figure S1. Checking the knockdown efficiency in T.castaneum larvae and cDNA library preparation for RNA seq. Figure S2. Normalization of RNA-seq data. Figure S3. Histogram presentation of GO ontology classification with 1306 genes that were downregulated in T.castaneum larvae after CBP knockdown. Figure S4. Epi-factor domains within the downregulated genes (1306) after CBP knockdown in T.castaneum larvae. Figure S5. KEGG pathway analysis. Figure S6. Correlation of gene expression levels of 20 selected genes by comparing both qPCR and RNA-seq data. Supporting Information S1. KEGG pathway analysis output. (DOCX 2490 kb)
Additional file 2:
Excel file S1. Details of 1306 genes downregulated by CBP RNAi. (XLSX 495 kb)
Additional file 3:
Excel file S2. Details of 52 genes identified after k-mer clustering. (XLSX 18 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Roy, A., George, S. & Palli, S.R. Multiple functions of CREB-binding protein during postembryonic development: identification of target genes. BMC Genomics 18, 996 (2017). https://doi.org/10.1186/s12864-017-4373-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12864-017-4373-3