Abstract
Background
Association studies in recently admixed populations are extremely useful to identify the genetic architecture of pigmentation, due to their high genotypic and phenotypic variation. However, to date only four Genome-Wide Association Studies (GWAS) have been carried out in these populations.
Results
We present a GWAS of skin pigmentation in an admixed sample from Cuba (N = 762). Additionally, we conducted a meta-analysis including the Cuban sample, and admixed samples from Cape Verde, Puerto Rico and African-Americans from San Francisco. This meta-analysis is one of the largest efforts so far to characterize the genetic basis of skin pigmentation in admixed populations (N = 2,104). We identified five genome-wide significant regions in the meta-analysis, and explored if the markers observed in these regions are associated with the expression of relevant pigmentary genes in human melanocyte cultures. In three of the regions identified in the meta-analysis (SLC24A5, SLC45A2, and GRM5/TYR), the association seems to be driven by non-synonymous variants (rs1426654, rs16891982, and rs1042602, respectively). The rs16891982 polymorphism is strongly associated with the expression of the SLC45A2 gene. In the GRM5/TYR region, in addition to the rs1042602 non-synonymous SNP located on the TYR gene, variants located in the nearby GRM5 gene have an independent effect on pigmentation, possibly through regulation of gene expression of the TYR gene. We also replicated an association recently described near the MFSD12 gene on chromosome 19 (lead variant rs112332856). Additionally, our analyses support the presence of multiple signals in the OCA2/HERC2/APBA2 region on chromosome 15. A clear causal candidate is the HERC2 intronic variant rs12913832, which has a profound influence on OCA2 expression. This variant has pleiotropic effects on eye, hair, and skin pigmentation. However, conditional and haplotype-based analyses indicate the presence of other variants with independent effects on melanin levels in OCA2 and APBA2. Finally, a follow-up of genome-wide signals identified in a recent GWAS for tanning response indicates that there is a substantial overlap in the genetic factors influencing skin pigmentation and tanning response.
Conclusions
Our meta-analysis of skin pigmentation GWAS in recently admixed populations provides new insights about the genetic architecture of this complex trait.
Similar content being viewed by others
Background
Constitutive human skin pigmentation (i.e. pigmentation of unexposed skin) is one of the most diverse phenotypes among worldwide populations. Previous work indicates that there has been selective pressure upon this phenotype at a global scale, with higher melanin levels favored in equatorial and tropical regions due to its protective effects against the harmful effects of ultraviolet radiation, and lighter skin pigmentation in higher latitudes, possibly because it facilitates cutaneous synthesis of vitamin D [1, 2].
Association studies in recently admixed populations can be extremely useful to identify the genetic architecture of pigmentation, due to their wide range of genotypic and phenotypic variation, and the increased statistical power to detect associations for genetic markers that have extreme frequencies in the parental population groups [3]. For instance, the identification of the genomic regions with the strongest effect reported on skin pigmentation (SLC24A5 and SLC45A2) was originally done in admixed individuals [4, 5]. Unfortunately, to date only four genome-wide pigmentation studies have been conducted in recently admixed populations [6,7,8,9]. Similarly, in spite of the fact that there is substantial variation in the skin pigmentation spectrum within the African continent, to date only two GWAS have been conducted in African populations [10, 11].
Here, we present the results of a GWAS of skin pigmentation in an admixed sample from Cuba [12]. Additionally, to increase statistical power, we carried out a meta-analysis including available genome-wide single-nucleotide polymorphism (SNP) data of four recently admixed samples: the Cuban sample, the Cape Verde sample [6], and the Puerto Rican and African American samples [8]. These samples are characterized by different patterns of admixture between European and West African populations. The overall sample includes 2,104 individuals. To our knowledge, this is one of the largest meta-analysis conducted so far for skin pigmentation in admixed populations. We also followed-up the genome-wide signals that have been described in previous GWAS in recently admixed samples and in samples from the African continent [10, 11].
Results
GWAS of skin pigmentation in the Cuban sample
The distribution of M-index in the Cuban sample has a wide range of M-index values, from the low 20s to higher melanin content than 80 (Additional file 1: Figure S1). On average, M-index was 39.88 (SD = 10.01). The top variant identified in the GWAS is rs35397 (p = 9.49 × 10− 13), located within the gene SLC45A2 on chromosome 5 (Additional file 1: Figure S2). Variants located within the gene SLC24A5 on chromosome 15 were also close to genome-wide significance, with the known non-synonymous rs1426654 variant being the top SNP in this region (p = 3.42 × 10− 7). A QQ plot of the GWAS p-values shows the observed vs. expected distribution, with deviations at the extreme tail of the distribution (λGC = 1.046; Additional file 1: Figure S3). Detailed information about the genome-wide (p < 10− 8) and suggestive signals (p < 10− 5) identified in the analysis is available in the Additional file 3.
Meta-analysis of association results in four admixed samples
We combined the summary statistics of the Cuban sample with three available datasets from Cape Verde [6], African Americans and Puerto Ricans [8]. All of the individual GWAS were carried out using the same analytical strategy and took into account population structure (i.e. individual admixture proportions, for more detailed information see Methods section). Prior to performing the meta-analysis, the standard errors of the beta coefficients were corrected based on the estimated lambda values. The ancestry estimates for each of the datasets are summarized in Table 1. There is a significant correlation of African individual proportions and melanin index in each of the samples, but there are clear differences in the percentage of skin pigmentation variation explained by ancestry in the Cuban (64.5%), Cape Verdean (43.6%), African-American (21.6%), and Puerto Rican (18.2%) sample (Additional file 1: Figures S4-S7).
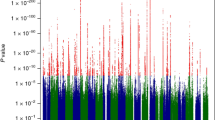
The Manhattan plot depicting the results of the meta-analysis is shown in Fig. 1, and the QQ plot is depicted in Additional file 1: Figure S8. As expected, both rs1426654 (SLC24A5) and rs35397 (SLC45A2) show very strong effects in the meta-analysis (rs1426654, p = 6.32 × 10− 39, and rs35397, p = 1.98 × 10− 24, respectively). The SNP with the second lowest p-value (p = 2.13 × 10− 23) in the SLC45A2 region is the non-synonymous variant rs16891982. Additional file 2: Table S1 provides information about all the genome-wide signals identified in the meta-analysis, including p-values, estimates of between-study heterogeneity (Cochran’s Q and corresponding p-value and I2 value), p-values and M-values of the individual studies, and allele frequencies of the tested alleles in the study populations, as well as African and European populations.
Given the very strong effects observed in the SLC24A5 and SLC45A2 regions, the meta-analysis was repeated after controlling for the polymorphisms rs1426654 and rs35397 in each study. Figure 2 depicts the Manhattan plot corresponding to this analysis, and the QQ plot (λGC = 1.016) is presented on Additional file 1: Figure S9. Further details about the genome-wide signals identified in this analysis are provided in Table 2 (meta-analysis results) and Additional file 2: Table S2 (meta-analysis results, and p-values and allele frequencies in each sample). The results with and without controlling for SLC24A5 and SLC45A2 are very consistent. Detailed information of the genome-wide and suggestive signals identified in the main meta-analysis and the meta-analysis controlling for SLC24A5 and SLC45A2 are available in the Additional file 3.
In addition to SLC24A5 and SLC45A2, we identified three regions surpassing the genome-wide significance threshold (Table 2). The first region includes multiple genome-wide significant markers overlapping with the GRM5 and TYR genes on chromosome 11. The most significant marker, rs10160510 (p = 3.36 × 10− 10), is located in an intronic region of the GRM5 gene. This marker shows evidence of effect size heterogeneity in the meta-analysis (Q p-value = 0.011). The second signal corresponds to an intergenic region between the genes MFSD12 and HMG20B on chromosome 19 and contains two genome-wide significant markers in close proximity to each other (~ 2 kb), the most significant SNP being rs10416746 (p = 1.55 × 10− 9). The third region comprises a large number of genome-wide significant variants on chromosome 15 spanning more than 1.6 megabases (from 27.8 Mb to 29.5 Mb in the GRCh37 human assembly). This region includes the well-known OCA2/HERC2 pigmentation genes, as well as the APBA2 gene previously reported by Beleza et al. [6]. We provide additional analyses of this region below.
Linkage disequilibrium, conditional and haplotype analyses of OCA2/HERC2/APBA2 region
There are at least four clusters of genome-wide significant variants, each of them separated by more than 250 Kb, in the 27.8–29.5 Mb interval on chromosome 15. We carried out a detailed analysis of linkage disequilibrium (LD) patterns between the genome-wide signals observed in the Cuban and Cape Verde samples, as well as the PUR and ASW samples. Additional file 1: Figures S10-S17 show in graphical format the r2 and D’ values between the genome-wide significant SNPs. Additionally, Additional file 1: Figures S18-S27 show the regional plots corresponding to this region, highlighting the LD patterns of the lead SNPs in each cluster in African and European populations.
The first cluster includes multiple genome-wide significant markers in strong LD upstream of the gene OCA2. The top SNP in this cluster is rs3098576 (p = 8.9 × 10− 9). Based on the r2 and D’ estimates, the markers in this cluster are not in strong LD (D’ < 0.5) with any of the lead SNPs located in the other clusters, including a cluster located approximately 400 Kb apart within the gene OCA2.
The second cluster includes markers located within the OCA2/HERC2 genes, from 28.28 Mb to 28.54 Mb. The most significant marker within OCA2 is rs1448484 (p = 3.73 × 10− 10), located in an intronic region of the gene. In HERC2, the top marker is rs1667392 (p = 4.64 × 10− 9). OCA2 rs1448484 and HERC2 rs1667392 are separated by approximately 250 Kb.
The third cluster corresponds to SNPs close to or within the gene APBA2. This cluster is located more than 550 Kb away from the OCA2/HERC2 cluster. The top marker in this region is rs36194177 (p = 4.95 × 10− 10). For this marker there is evidence of effect size heterogeneity in the meta-analysis (Q p-value = 0.026).
The fourth cluster is located within the gene FAM189A1, which is located approximately 270 Kb away from the APBA2 cluster. The top SNP in this region is rs2636060, (p = 5.71 × 10− 10).
As shown in Additional file 1: Figures S10-S17, although the r2 values between the markers located in different clusters are lower than 0.5 in all the studied populations, the D’ values, which are not as influenced by allele frequencies, indicate that the markers located in clusters 2–4 are in linkage disequilibrium (D’ > 0.8 in many inter-marker comparisons). For this reason, we also carried out conditional tests and association analyses at the haplotype level in the samples of Cape Verde and Cuba.
We first performed association tests conditioning for each of the lead signals in this region: rs1448484 (OCA2), rs1667392 (HERC2), rs36194177 (APBA2) and rs2636060 (FAM189A1). The results of these analyses are reported in Additional file 2: Table S3. As expected given the patterns of LD observed in the Cape Verde and Cuban samples, conditioning for the lead SNPs in the OCA2/HERC2/APBA2 region decreases the beta estimates and the p-values of the other markers. However, in the Cape Verde sample all of the markers remain nominally significant after conditioning, except for rs1448484 after conditioning for rs2636060.
We also carried out association tests based on haplotypes for rs1448484, rs1667392, rs36194177, and rs2636060. The results of the haplotype association tests for the Cape Verde and Cuban sample are shown in Additional file 2: Tables S4 and S5, respectively. The beta values report the effect sizes estimated for each haplotype, using the haplotype GGAG as the reference. This haplotype contains the alleles that are common in African populations and are in all cases associated with higher melanin index values in the meta-analysis.
In the Cape Verde sample, the omnibus haplotype test is significant (p = 1.38 × 10− 6) and the amount of skin pigmentation variation explained by the model is 6.3%. The haplotype with the strongest effect is the ACGA haplotype, which includes all the alleles common in European populations (beta = − 0.466). Other haplotypes associated with lower melanin levels are GGGA (beta = − 0.326), AGGA (beta = − 0.268) and AGGG (beta = − 0.229). In the sample from Cuba, the omnibus test is also significant (p = 0.0131) and the haplotype model explains 2.73% of the skin pigmentation variation. In this sample, the results are quite consistent with those observed in the Cape Verde sample. The haplotypes showing the strongest effects on pigmentation are AGGG (beta = − 0.330), ACGA (beta = − 0.322) and GGGA (beta = − 0.202).
In addition to the omnibus tests, we also carried out tests controlling for the haplotypes with the strongest effects, to explore if a single haplotype can explain the observed effects. In the sample of Cape Verde, after controlling for the haplotype ACGA, which has all the alleles common in European populations, there is still a marginal association (p = 0.0182), as is the case after controlling for ACGA and GGGA (p = 0.0204). Controlling for ACGA, GGGA and AGGA, the model is not significant. In the Cuban sample, after controlling for ACGA, the results are not significant.
Association of top meta-analysis variants with gene expression data
We evaluated the potential association of the genome-wide significant signals identified in the meta-analysis with gene expression, using transcriptome and genotype data from primary cultures of human melanocytes isolated from foreskin of more than 100 individuals of diverse ancestries [13]. Table 3 shows the strength of association of the meta-analysis top signals with the expression of key pigmentation genes (SLC45A2, TYR, OCA2 and SLC24A5), and for comparative purposes, also reports the variants in each region with the strongest association to gene expression, and their respective p-values in the meta-analysis. The two markers on chromosome 5 showing the strongest associations with melanin levels in the meta-analysis are among the variants with the strongest effects on SLC45A2 expression (nominal p-values for SLC45A2 expression, rs35397, p = 3.95 × 10− 5, and rs16891982, p = 4.78 × 10− 7). In the OCA2/HERC2/APAB2 region, five of the genome-wide signals were associated with expression of the key pigmentary gene OCA2. Four of these variants are located in the HERC2 gene (nominal p-values for OCA2 expression, rs12913832, p = 3.14 × 10− 23; rs1129038, p = 1.24 × 10− 22; rs6497263, p = 6 × 10− 3; and rs73377768, p = 6.6 × 10− 3). The variant rs1448484, which is located in the OCA2 gene, was also nominally associated with OCA2 expression (p = 0.022). It is important to note that several of the genome-wide signals identified in the OCA2/HERC2/APAB2 region, including some variants within or near the HERC2, APAB2 and FAM189A1 genes, were not present in the expression quantitative trait loci (eQTL) dataset, due to poor imputation quality or low allele frequency (minor allele frequency (MAF) < 0.01), so it was not possible to evaluate if these variants are associated with gene expression. Similarly, the two genome-wide significant variants identified in the chr19 region were not present in this dataset.
In the GRM5/TYR region on chromosome 11, multiple genome-wide variants located within the GRM5 gene (e.g. rs12801588, rs10160510 and rs10437581) are nominally associated with the expression of the TYR gene (Table 3). However, the variants identified to have the strongest association with the TYR gene in the eQTL study (rs28437494, rs4121729 and rs7925346), which are located more than 200 Kb apart from the aforementioned GRM5 variants, are not nominally significant in the meta-analysis. Regarding the SLC24A5 region, none of the genome-wide signals observed in the meta-analysis for this region were strongly associated with expression of the SLC24A5 gene (all p-values were higher than 0.01 in this region). Of note, the non-synonymous variant rs1426654 was present in the imputed dataset with a frequency of 12% and high imputation confidence (r2 = 0.99948), but it was not associated with expression of SLC24A5 (p = 0.218).
Replication of lead signals of meta-analysis in East African sample
We replicated the lead SNPs identified in the meta-analysis in an East African sample (Table 4) [10]. The direction of effects is concordant for all the polymorphisms, except for rs3098576, which is located upstream of OCA2 on chromosome 15. The markers rs1667392 (HERC2) and rs36194177 (APBA2) were not present in the imputed dataset. The polymorphism rs1426654 in SLC24A5 is also genome-wide significant in the East African sample (p = 6.89 × 10− 61), and two additional markers are nominally significant (rs1448484 in OCA2, p = 4.43 × 10− 4 and rs10416746 in MFSD12, p = 1.53 × 10− 4). The results of the follow-up for all the genome-wide signals are presented in Additional file 2: Table S6, providing additional relevant information. The second most significant SNP observed in the meta-analysis in the MFSD12 region, rs112332856, reaches genome-wide significance in the East African sample (p = 1.15 × 10− 15). Additionally, many of the genome-wide signals identified in the GRM5 gene in the meta-analysis, which have very low frequencies in the East African sample (less than 2%), are nominally significant and have strong effects on melanin levels. The lowest p-value correspond to the SNP rs35790407 (p = 3.87 × 10− 3, beta allele A = − 5.501). Unfortunately, some of the key variants in this region, including the non-synonymous SNP rs1042602 located in the TYR gene, are not present in the imputed East African dataset. Finally, two of the variants located in the APBA2 region, rs148942115 and rs150641715, are nominally significant in the East African sample (rs150641715 p = 0.017, beta allele A = − 0.955).
Follow-up of genome-wide signals described in recent studies
We also followed-up in our meta-analysis the genome-wide signals reported in the original GWAS in recently admixed populations (i.e. the samples that were included in this meta-analysis), as well as the recent GWAS in East and South African populations (Table 5) [10, 11]. Concordant effects were observed in all the previously described regions. For SLC24A5, SLC45A2, and MFSD12, at least some of the original signals described are also genome-wide significant in our meta-analysis. The lead signal reported in the GRM5 gene [6] is also very close to genome-wide significance in our meta-analysis.
In the OCA2/HERC2/APBA2 region, the original signals reported in Cape Verde and East Africa do not reach genome-wide significance. As described above, we have observed other variants in this region that reach genome-wide significance in our meta-analysis. In the DDB1/TMEM138 region, several of the lead SNPs reported in a recent Bayesian analysis of the Cape Verde sample [7], and a recent East African GWA study [10] are nominally significant in our meta-analysis. These results are primarily driven by the Cape Verde sample, and the p-values in the other samples are not significant. We could not confirm the genome-wide signals identified in the BEND7/PRPF18 region in Puerto Rican and African-American samples [8] and the LOC102724084 region in the Cuban sample. Finally, we also followed-up in our meta-analysis five suggestive variants reported in the recent GWA study in KhoeSan [11]: rs7866411 and rs2093835 in the SMARCA2/VLDLR region, rs34803545 and rs76413115 upstream of TYRP1, and rs210015 in SNX13. None of these SNPs was nominally significant in our meta-analysis.
Follow-up of genome-wide signals associated with tanning response
We followed-up 20 genome-wide signals recently identified in a meta-analysis of tanning response including five large European samples [14] (Additional file 2: Table S6). Five variants located in known pigmentation genes are nominally significant in our meta-analysis: rs16891982 (SLC45A2; p-value = 2.13 × 10− 23), rs12203592 (IRF4; p-value = 1.4 × 10− 3), rs72917317 (TPCN2; p-value = 4.87 × 10− 4), rs1126809 (TYR; p-value = 1.73 × 10− 3), and rs12913832 (HERC2; p-value = 3.18 × 10− 8). One of the novel markers identified in the tanning response meta-analysis is also nominally significant in our conditional meta-analysis: rs35563099 (EMX2; p-value = 2.0 × 10− 3).
Discussion
Here, we present the results of one of the largest association studies of skin pigmentation in recently admixed populations. Our meta-analysis includes over 2,000 unrelated admixed individuals from Cuba, Cape Verde, Puerto Rico and African Americans from the San Francisco Bay area. Although there are significant associations of African ancestry with skin pigmentation in all samples (Additional file 1: Figures S4-S7), there are considerable differences in the strength of the association, with R2 values ranging from 0.645 in the Cuban sample to 0.182 in the Puerto Rican sample. This is probably driven by differences in the population structure present in the samples. For the meta-analysis, we used summary data obtained after controlling for population structure (i.e. individual ancestry) and other relevant covariates in each sample.
The strongest signals observed in the meta-analysis correspond to SNPs in the well-known SLC24A5 and SLC45A2 genes. In both genes, the best candidates to explain the association are two non-synonymous variants: rs1426654 (SLC24A5) and rs16891982 (SLC45A2). The polymorphism rs16891982 is in very strong LD (r2 > 0.8) with another SNP in the SLC45A2 gene, rs35397, which is the lead signal in our meta-analysis. The SNP rs16891982 is predicted to be damaging by both SIFT and Polyphen, so its effect may be mediated by structural changes in the protein coded by the SLC45A2 gene, the membrane associated transporter protein (MATP), which works as a membrane transporter in melanosomes. Additionally, our eQTL analyses indicate that rs16891982 is associated with expression of the SLC45A2 gene (p = 4.78 × 10− 7), although we cannot exclude the possibility that this association is driven by potential LD with other variants not genotyped in this region. The derived G allele, which is associated with lower melanin levels, is associated with an increased expression of the SLC45A2 gene. SLC24A5 rs1426654 and SLC45A2 rs16891982 are the variants with the strongest effect on skin pigmentation described in human populations and have been reported in numerous association studies [4,5,6,7,8,9,10,11, 15,16,17]. Further, SLC45A2 rs16891982 has also been associated with tanning response and skin cancer in several studies [15, 18,19,20].
Genome-wide association signals were also identified in a region encompassing the genes GRM5 and TYR on chromosome 11. Another clear candidate to explain this association is the non-synonymous rs1042602 located in the TYR gene (p = 9.2 × 10− 10), which is in strong LD (r2 > 0.70) with our lead SNP (rs10160510, p = 3.36 × 10− 10) and it is predicted to be damaging by both SIFT and Polyphen. Enhancer histone marks, H3K4me1 and H3K27ac, have been identified in foreskin melanocyte primary cells overlapping two variants that are in high LD with rs1042602: rs12285584 and rs12295166. Two GWAS have previously reported associations of rs1042602 with pigmentary traits, such as skin pigmentation [21] and freckles [22]. Importantly, there seem to be more than one independent signal in the GRM5/TYR region. When carrying out association tests in the Cape Verde sample conditioning for rs1042602, or rs10160510, there are several genome-wide significant markers in the GRM5 gene that remain strongly associated with skin pigmentation (e.g. rs492312, p-value conditioning for rs1042602 = 3.82 × 10− 6, p-value conditioning for rs10160510 = 1.65 × 10− 4). The SNP rs492312 is in strong LD (r2 > 0.90) with markers that overlap with enhancer histone marks and/or DNase hypersensitive sites in foreskin fibroblast primary skin cells [23]. We also found support for the role of variants within the GRM5 gene in the follow-up of our genome-wide significant signals in the East African sample (Additional file 2: Table S6). Many of these variants, although found at very low frequencies in the East African sample (less than 2%), are nominally associated with skin pigmentation, and the estimated effects on skin pigmentation are very large (e.g. rs35790407, MAF = 1.1%, p = 3.87 × 10− 3, beta = − 5.501; rs11021131, MAF = 1.6%, p = 0.012, beta = − 3.957). We hypothesize that the association of variants located on the GRM5 gene with pigmentation may be due to their regulation of expression of the TYR gene, which is a critical gene in melanogenesis.
We also observed strong associations of variants located on chromosome 19, near the MFSD12 gene, with skin pigmentation. The role of this gene on skin pigmentation has only been recently described. Crawford et al. [10] reported variants associated with skin pigmentation in two different regions on chromosome 19, one within and another upstream of the MFSD12 locus, in an East African sample. Additionally, they showed, using experiments in mouse melanocyte cultures and zebrafish and mice animal models, that MFSD12 has a functional role on pigmentation. Of note, one of the SNPs identified in our meta-analysis, rs112332856 (2.31 × 10− 8), is one of the genome-wide variants originally reported by [10]. This SNP is associated with expression of the MFSD12 gene. In particular, the rs112332856 C allele, which is associated with darker skin pigmentation, is associated with decreased expression of MFSD12, and this is consistent with experiments in mouse melanocyte culture that indicate that depletion of MFSD12 increases eumelanin biogenesis in melanocytes.
Additionally, we observed multiple genome-wide signals in a broad region of chromosome 15 overlapping the genes OCA2/HERC2/APBA2. A cluster of variants is located upstream of the OCA2 gene (lead SNP rs3098576, p = 8.9 × 10− 9) and the markers in this region are not in strong LD with other genome-wide signals identified in the OCA2/HERC2/APBA2 region. The posterior probabilities of having an effect (M-values) are high in all the samples of the meta-analysis, and there is no evidence of heterogeneity in effect sizes. However, we could not replicate these results in the East African sample, in spite of the fact that the relevant variants are present at high frequencies in the sample. It will be necessary to carry out additional studies to elucidate the potential role of this region on skin pigmentation variation.
Aside from the aforementioned region, we identified genome-wide clusters in a segment of approximately 1 Megabase that includes markers located within the OCA2 gene, the HERC2 gene and within or nearby the APBA2 and FAM189A1 genes. These clusters are located more than 250 Kb apart, but the markers in different clusters are in LD (D’ > 0.8; Additional file 1: Figures S10-S17). Identifying the putative causal variants in this region is challenging for two reasons: the aforementioned LD between markers, and problems with imputation of variants between the HERC2 and APBA2 region, which is probably due to the presence of segmental duplications in this genomic region [24]. For example, some of the genome-wide signals identified in our meta-analysis were not present in the imputed data used to evaluate gene expression in melanocytes, or in the imputed East African dataset.
In spite of these challenges, there are several functional candidates in the OCA2/HERC2/APBA2 region. Within OCA2, our lead SNP is rs1448484. This variant has been identified in studies of signatures of selection as one of the markers with the highest continental differentiation in human populations [25, 26]. The derived G allele, which is strongly associated with light skin pigmentation, is almost fixed in non-African populations, whereas the ancestral A allele is the most common allele in African populations. This polymorphism has been associated with skin pigmentation in a previous study [27]. Additionally, this marker and linked OCA2 polymorphisms overlap with enhancer histone marks and DNase hypersensitive sites in foreskin fibroblast primary skin cells [23]. In the recent GWA study in East Africa, the main signal in the OCA2 region was the synonymous SNP rs1800404. However, this SNP is not in very strong LD with rs1448484 (r2 < 0.60), and is not genome-wide significant in our meta-analysis (rs1800404 p = 8.92 × 10− 6 vs. rs1448484 p = 3.73 × 10− 10). The high M-values observed in the meta-analysis indicate that the probabilities of having an effect are high for rs1448484 in the four samples analyzed. It would be important to carry out additional studies in substantially larger admixed and African samples in order to explore the reasons for the different association patterns identified in the OCA2 region.
Within HERC2, our lead signal rs1667392 (p = 4.64 × 10− 9) is in strong LD with rs12913832 (p = 3.18 × 10− 8), an intronic SNP that has a known regulatory effect on OCA2 expression by disrupting the interaction between an enhancer located on HERC2 and the OCA2 promoter [28, 29]. This SNP is strongly associated with blue eye color [28,29,30,31,32,33]. Our melanocyte eQTL data fully supports the functional effects described for rs12913832; the derived G allele, which is associated with light pigmentation in our meta-analysis, shows a strong association with reduced OCA2 expression (p = 3.14 × 10− 23). Of note, in addition to the extensively reported association with blue eye color, rs12913832 has been associated with skin pigmentation and hair color in previous studies [15, 22, 34,35,36,37].
We also observed genome-wide significant signals downstream of HERC2, near the APBA2 gene. This region was previously reported in the original GWAS in the Cape Verde sample [6], although the top signals reported in that study are not the same as those identified in our meta-analysis. Interestingly, the lead SNP in the meta-analysis (rs36194177, p = 4.95 × 10− 10), and variants in LD with this SNP (rs142415892 and rs148942115), overlap with enhancer histone marks and DNase hypersensitive sites in several tissues, and there is an enrichment for enhancers in foreskin keratinocyte primary cells in skin [23]. Unfortunately, none of our genome-wide signals in the APBA2 region was present in the imputed data used to evaluate gene expression in melanocyte cultures, so we could not evaluate potential associations of these variants with gene expression of relevant genes, and more particularly, OCA2. However, three of the variants were present in the East African imputed dataset (rs142415892, rs148942115, and rs150641715), and two of them are nominally associated with skin pigmentation (e.g. rs150641715, p = 0.017).
The final genome-wide signals identified in this region are located within the FAM189A1 gene (lead SNP rs2636060, p = 5.7 × 10− 10). To our knowledge, this region has not been described in previous pigmentation association studies. The posterior probabilities of having an effect (M-values) are high in all the samples of the meta-analysis, and there is no evidence of heterogeneity in effect sizes, as observed for the region located upstream of OCA2. However, the two genome-wide signals are not significant in the East African sample, and there is no overlap of this region with putative regulatory regions active in skin cells [23].
Given the pattern of LD observed in the OCA2/HERC2/APBA2 region, we carried out additional conditional and haplotype tests to evaluate if the signals observed in the meta-analysis may be due to a single causal SNP, or to independent effects. These tests point to the presence of independent variants. When conditioning for each of the lead SNPs from the four relevant clusters, rs1448484 (OCA2), rs1667392 (HERC2), rs36194177 (near APBA2) and rs2636060 (FAM189A1), the p-values of the other markers remain nominally significant. The haplotype tests also support at least partially independent effects. As expected, the haplotype associated with the strongest reduction in melanin levels is the haplotype harboring the four variants common in European populations (ACGA). However, there are other haplotypes that do not include some of the variants common in Europe that are also associated with lower melanin levels, and the haplotype patterns are quite similar in Cape Verde and Cuba. Controlling for the haplotype ACGA in the Cape Verdean sample, there is still a marginal association with skin pigmentation, which is driven by the haplotypes GGGA and AGGA.
Given that some variants that have an effect on constitutive pigmentation have been found to be associated with facultative pigmentation (i.e. tanning ability) [14, 18], we followed-up in our meta-analysis the genome-wide significant markers identified in a recent tanning response GWAS [14]. These results are depicted in Additional file 2: Table S7. One of the novel markers identified in the aforementioned GWAS, located nearby EMX2 on chromosome 10 is nominally significant in our meta-analysis of constitutive pigmentation (rs35563099, p-value = 0.002). EMX2 is known to be involved in the synthesis of melanin, and it has been shown that overexpression of the Emx2 homologous gene causes loss of pigmentation by downregulating the Mitf gene in mice [38]. Our study indicates that this gene is involved both in tanning response and constitutive skin pigmentation.
When our manuscript was under review, an article was published describing the results of a GWAS of skin and iris pigmentation in a sample of more than 6,000 Latin Americans [9]. Some of the findings of this study are very similar to those described in the present study. More particularly, the authors also reported multiple independent signals within the GRM5/TYR and OCA2/HERC2 regions. In the GRM5/TYR region, Adhikari et al. [9] reported three signals: rs7118677 (GRM5), rs1042602 (TYR) and rs1126809 (TYR). In the OCA2/HERC2 region, they reported five signals: rs4778219 (OCA2), rs1800407 (OCA2), rs1800404 (OCA2), rs12913832 (HERC2) and rs4778249 (HERC2). We followed up all the genome-wide signals reported by Adhikari et al. [9] in our meta-analysis (Additional file 2: Table S8). Several of these signals are also the lead signals identified in our study (e.g. rs1042602 in TYR, rs12913832 at HERC2), clearly pointing to the same causal variants. However, other lead variants are different to our lead SNPs in the relevant regions (Table 2; Additional file 2: Table S8), and Adhikari et al. [9] do not report genome-wide significant variants within the APBA2 region. Some of these discrepancies may be driven by the demographic differences between both datasets. The GWAS on Latin Americans [9] includes samples from Chile, Mexico, and Peru, which have primarily Native American and European ancestry, with very small African ancestral contributions (< 5%), and samples from Brazil and Colombia, which have primarily European ancestry, with substantially lower Native American (12.1 and 29.2%, respectively) and African contributions (~ 10% in both samples). In contrast, the samples included in our study are characterized by a wide distribution of West African and European ancestry, without (e.g. Cape Verde) or with very small Native American ancestry (Cuba, Puerto Rico, African Americans).
Interestingly, Adhikari et al. [9] also reported a non-synonymous genome-wide signal within the gene MFSD12: rs2240751. However, this missense variant is common only in East Asian and Native American populations, and is different from the variants reported in the recent GWAS in East African populations [10] and those we found in the present study, which reflects the different demographic composition of the samples. Clearly, there have been independent mutational events within this gene in African and East Asian/Native American groups.
In summary, in this meta-analysis we have identified multiple regions significantly associated with skin pigmentation variation in admixed populations. All of these regions have been reported before. However, our analyses indicate that in some of these regions there are multiple independent signals. We have not identified any novel pigmentation regions in our study. This may be related to limited power to identify variants with moderate effects on skin pigmentation. Further meta-analytic efforts including larger sample sizes will be needed to uncover this type of variants, and to elucidate the reasons driving the heterogeneity in effect sizes observed for some of the signals identified in this study.
Conclusions
We identified five genome-wide significant regions in a meta-analysis of skin pigmentation in recently admixed populations with primarily European and African ancestry, and explored if the markers observed in these regions are associated with the expression of relevant pigmentary genes in human melanocyte cultures. In three of the regions identified in the meta-analysis (SLC24A5, SLC45A2, and GRM5/TYR), the association seems to be driven by non-synonymous variants (rs1426654, rs16891982, and rs1042602, respectively). The rs16891982 polymorphism is strongly associated with the expression of the SLC45A2 gene. In the GRM5/TYR region, in addition to the rs1042602 non-synonymous SNP located on the TYR gene, variants located in the nearby GRM5 gene have an independent effect on pigmentation, possibly through regulation of gene expression of the TYR gene. We were also able to confirm the effect of a recently described variant near the MFSD12 gene (rs112332856) on skin pigmentation. This variant has been shown to alter the expression of the nearby gene [10]. Finally, our analyses support the presence of several variants with effects on skin pigmentation in the OCA2/HERC2/APBA2 region on chromosome 15. A clear causal candidate is rs12913832 within HERC2, which has a profound influence on OCA2 expression in eye, hair, and skin pigmentation. However, our results also point out the presence of other independent variants modulating melanin levels in the genes OCA2 and APBA2.
Overall, our meta-analysis and other GWAS in multiple population groups provide fascinating insights on the genetic architecture and evolutionary history of pigmentary traits. 1) There is evidence of independent functional mutations within the same genes in different populations (i.e. convergent evolution). Different variants within OCA2/HERC2 are associated with light pigmentation in European and East Asian populations [39,40,41,42]. Independent mutations within the MFSD12 gene are associated with skin pigmentation variation in populations with diverse African ancestry and Native American/East Asian ancestry (e.g. this study, [9, 10]). 2) There is evidence of multiple independent signals in the same genomic regions, such as GRM5/TYR and OCA2/HERC2, within the same population group (e.g. this study, [9]). 3) There is evidence of pleiotropic effects for many significantly associated variants. For example, the HERC2 rs12913832 SNP, which was originally associated with eye color, also plays a role in skin and hair color pigmentation [15, 22, 34,35,36,37]. Additionally, many of the variants associated with skin pigmentation are also associated with tanning response (e.g. EMX2) (this study, [9, 14]). Unfortunately, studies focusing on tanning response are still in their infancy. 4) Finally, there is evidence pointing to the mechanisms of action by which genetic polymorphisms exert a role on pigmentation variation (e.g. effects on protein structure, regulation of gene expression).
As new studies emerge with increasing sample sizes and more comprehensive population coverages, it will be possible to gain an even deeper understanding of the main events that shaped the diversity of pigmentary traits and allowed our species to adapt to different latitudinal environments.
Methods
Cuban dataset
The Cuban sample comprises 1,019 individuals representing all Cuban provinces [12]. Skin pigmentation was measured using the DSM II Derma Spectrometer (Cortex Technology, Hadsund, Denmark) in triplicate for each participant, from the inner skin of their upper right arm. The DNA samples were genotyped using the Infinium PsychChip v1.0 and v1.1 arrays (Illumina Inc., San Diego, California, U.S.A.), at the Statens Serum Institut in Copenhagen, Denmark. We performed quality control (QC) steps to remove samples and markers, according to the following criteria, Sample QC: 1/removal of samples with missing call rates < 0.95, 2/removal of samples that were outliers in Principal Component Analysis (PCA) plots, 3/removal of duplicates or samples with sex discrepancies, 4/removal of samples that were outliers for heterozygosity, and 5/removal of related individuals (pi-hat > 0.2). Marker QC: 1/removal of markers with genotype call rate < 0.95, 2/removal of markers with Hardy-Weinberg p-values < 10− 6, 3/removal of Insertion/Deletion (Indel) markers, 4/removal of markers with allele frequencies < 0.01, 5/removal of markers not present in the 1000 Genomes reference panel, or that do not match on chromosome, position and alleles, 6/removal of A/T or G/C SNPs with MAF > 40% in the 1000 Genomes American (AMR) samples, and 7/removal of SNPs with allele frequency differences > 20% between the study sample and the 1000 Genomes AMR reference sample. The final call set includes a total of 292,549 autosomal SNPs across 762 unrelated Cuban individuals with skin pigmentation data. Haplotype phasing was done with the program SHAPEIT2 [43]. Imputation of non-genotyped SNPs was done at the Sanger Imputation Service, using the Positional Burrows-Wheeler Transform algorithm [44], and the samples of the 1000 Genomes Project Phase 3 (1KGP) as reference haplotypes.
Datasets of other admixed samples
We carried out a meta-analysis based on the GWAS results for the Cuban sample, as well as imputed genome-wide data available for three additional admixed samples. The first sample comprises 684 individuals from the archipelago of Cape Verde [6]. The second sample includes 373 African Americans from the San Francisco Bay Area, who were recruited as part of the Study of African Americans, Asthma, Genes and Environments (SAGE II) [8]. The third sample comprises 285 unrelated individuals from Puerto Rico, who participated in the study of Genes-environment and Admixture in Latin Americans (GALA II) [8]. Further details for each dataset, such as information about skin pigmentation measurements, microarray genotyping and imputation procedure, are provided in Additional file 4: Text S1.
Statistical analyses
Inverse normal transformation
In order to combine the GWAS results of the four samples in a meta-analysis, quantitative pigmentation measures (M-index values) were transformed prior to statistical analysis using the rank-based inverse normal transformation (Blom method). In a first step, a linear regression was carried out using pigmentation values as the dependent variable, and sex, age, and relevant PCA scores as independent variables. The residuals of this regression were then transformed using the inverse normal transformation, which was used as outcome variable for the association tests.
Association tests
For the Cuban and Cape Verde samples association tests were performed in SNPTEST v2 [45], using expected genotype counts (e.g. genotype dosages) and an additive model. For the SAGE II and GALA II samples, association tests were performed with a linear Wald test (q.lm) implemented in the software EPACTS 3.2.6 [46] using genotype dosages and an additive model. Two sets of association analysis were done in each sample: standard association tests, and also association tests conditioning for the variants rs1426654 (SLC24A5) and rs35397 (SLC45A2), which are known to have very strong effects on pigmentation and had very low p-values in the four samples analyzed.
Meta-analysis of association results
The summary statistics from the four GWAS were used to run a meta-analysis using the program METASOFT [47, 48]. This program implements a fixed effects model based on inverse-variance-weighted effect size and also Han and Eskin’s random effects model (RE2), which has been shown to have higher statistical power to detect associations under heterogeneity than the conventional random effects model based on inverse-variance-weighted effect size [47]. Additionally, METASOFT provides estimates of the posterior probability that an effect exists in each study (M-values) [47]. Small M-values indicate that the study is predicted to not have an effect, large M-values indicate that the study is predicted to have an effect, and intermediate M-values indicate ambiguous results.
Linkage disequilibrium, conditional and haplotype analyses
To gain a better understanding of the multiple clusters identified in the OCA2/HERC2/APBA2 region on chromosome 15, we used the software Haploview [49], to explore the LD patterns (r2 and D’ values) between each pair of genome-wide signals found within this region in the Cuban and Cape Verde samples, as well as the Puerto Rican (PUR) and African American in Southwest US (ASW) samples included in the 1KGP.
To test whether the lead signals in each cluster are independent from each other, we carried out conditional analyses for this region in the Cuban and Cape Verde samples. Specifically, we conducted several association tests conditioning for each of the lead signals identified in each cluster. The genetic markers used were rs1448484 in OCA2, rs1667392 in HERC2, rs36194177 in APBA2, and rs2636060 in FAM189A1.
Additionally, haplotype association analyses were conducted in the Cuban and Cape Verde samples using the program PLINK v1.07 [50], which are the samples for which we had individual genome-wide data. Specifically, we employed omnibus tests to evaluate if there are significant effects of the haplotypes on the phenotype. These tests also provide estimates of effect sizes with respect to a reference haplotype. We defined the haplotype GGAG as the reference, given that it contains the alleles that are most frequent in African populations, which are all associated with higher melanin index values in the meta-analysis. Finally, we carried out haplotype tests controlling for specific haplotypes using PLINK –chap –control option. Again, these analyses were done based on haplotypes including rs1448484 in OCA2, rs1667392 in HERC2, rs36194177 in APBA2, and rs2636060 in FAM189A1.
Testing association with gene expression based on RNA-seq data
To test for associations between gene expression and genetic variation, we carried out an eQTL analysis based on transcriptome and genotype data from primary cultures of human melanocytes, isolated from foreskin of 106 healthy newborn males [13]. DNA samples were genotyped on Illumina OmniExpress arrays and genotypes were subsequently imputed using the Michigan Imputation Server [51], based on the 1KGP Phase 3 reference panel and using SHAPEIT for phasing [43]. Post-imputation variants with MAF < 0.01 or imputation quality scores (R2) < 0.3 were removed from the final analysis. PCA was performed to capture population structure using the struct.pca module of Genotyping Library and Utilities [52]. Analysis with the program ADMIXTURE indicated that 84 individuals are of predominant European ancestry, 3 individuals of predominant African ancestry, 3 individuals of predominant East Asian ancestry, 12 individuals show mixed European and African ancestry, and 4 individuals have mixed European and Asian ancestry [13]. RNA sequencing was performed on a HiSeq2500 using version 4 chemistry to achieve a minimum of 45 million 126 base paired reads. The updated pipeline originally published by University of North Carolina for processing TCGA RNA-sequencing data was used to analyze the RNA- sequence data generated from the samples. STAR [53] was used for aligning reads. RSEM [54] was used to quantify the gene expression to transcripts per million (TPM) and then quantile normalization was applied to the TPM in all samples to get the final RSEM value. To account for hidden factors driving expression variabilities, a set of covariates were further identified using the PEER method [55] and applied to calculate the normalized expression matrices. Associations between variant genotypes and gene expression levels were evaluated using linear regression implemented in FastQTL [56], using the set of covariates identified with the PEER method and the PC scores as covariates. Genetic variants located within +/− 1 Mb of the transcription start sites for each gene were tested for cis-eQTL effects of the corresponding gene. More detailed information about the gene expression analysis is available in [13].
Annotation of genome-wide association signals identified in the meta-analysis
The genome-wide association signals identified in the meta-analysis were annotated using the SNPnexus website [57]. This site provides numerous annotations, including potential effects on protein function (e.g. SIFT and PolyPhen), conservation scores (e.g. phastCons and GERP), and a range of scores for non-coding variants (e.g. CADD, fitCons, EIGEN, FATHMM, GWAVA, DeepSEA, FunSeq2, and ReMM). We also explored potential regulatory effects in HaploReg v4 [58] and RegulomeDB [59].
Availability of data and materials
We provide the list of all genome-wide (p < 5 × 10− 8) and suggestive (p < 10− 5) signals identified in the meta-analysis as a supplementary information file (Additional file 3). The complete summary data of the meta-analysis is available from the corresponding author upon request.
Abbreviations
- 1KGP:
-
1000 genomes project
- eQTL:
-
Expression quantitative trait loci
- GALA II:
-
Genes-environment and Admixture in Latin Americans
- GWAS:
-
Genome-wide association studies
- LD:
-
Linkage disequilibrium
- MAF:
-
Minor allele frequency
- SAGE II:
-
Study of African Americans, Asthma, Genes and Environment
- SNP:
-
Single nucleotide polymorphism
References
Jablonski NG, Chaplin G. Human skin pigmentation as an adaptation to UV radiation. Proc Natl Acad Sci U S A. 2010;107:8962–8. https://doi.org/10.1073/pnas.0914628107.
Jablonski NG, Chaplin G. The colours of humanity: the evolution of pigmentation in the human lineage. Philos Trans R Soc B Biol Sci. 2017;372:20160349. https://doi.org/10.1098/rstb.2016.0349.
Deng L, Xu S. Adaptation of human skin color in various populations. Hereditas. 2017;155:1–12.
Lamason RL, Mohideen MPK, Mest JR, Wong AC, Norton HL, Aros MC, et al. SLC24A5, a putative cation exchanger, affects pigmentation in Zebrafish and humans. Science (80- ). 2005;310(December):1782–6.
Norton HL, Kittles RA, Parra E, McKeigue P, Mao X, Cheng K, et al. Genetic evidence for the convergent evolution of light skin in Europeans and East Asians. Mol Biol Evol. 2007;24:710–22.
Beleza S, Johnson NA, Candille SI, Absher DM, Coram MA, Lopes J, et al. Genetic architecture of skin and eye color in an African-European admixed population. PLoS Genet. 2013;9(3):e1003372.
Lloyd-Jones LR, Robinson MR, Moser G, Zeng J, Beleza S, Barsh GS, et al. Inference on the genetic basis of eye and skin color in an admixed population via Bayesian linear mixed models. Genetics. 2017;206(June):1113–26.
Hernandez-Pacheco N, Flores C, Alonso S, Eng C, Mak ACY, Hunstman S, et al. Identification of a novel locus associated with skin colour in African-admixed populations. Sci Rep. 2017;7(October 2016):44548. https://doi.org/10.1038/srep44548.
Adhikari K, Mendoza-Revilla J, Sohail A, Fuentes-Guajardo M, Lampert J, Chacón-Duque JC, et al. A GWAS in Latin Americans highlights the convergent evolution of lighter skin pigmentation. Nat Commun. 2019;10:1–16.
Crawford NG, Kelly DE, Hansen MEB, Beltrame MH, Fan S, Bowman SL, et al. Loci associated with skin pigmentation identified in African populations. Science. 2017;358(6365). https://doi.org/10.1126/science.aan8433.
Martin AR, Lin M, Granka JM, Myrick JW, Liu X, Sockell A, et al. An unexpectedly complex architecture for skin pigmentation in Africans. Cell. 2017;171:1340–1353.e14. https://doi.org/10.1016/j.cell.2017.11.015.
Fortes-lima C, Bybjerg-grauholm J, Marin-padrón LC, Gomez-cabezas EJ, Bækvad-hansen M, Hansen CS, et al. Exploring Cuba’s population structure and demographic history using genome-wide data. Sci Rep. 2018;8:1–13.
Zhang T, Choi J, Kovacs MA, Shi J, Xu M, Goldstein AM, et al. Cell-type specific eQTL of primary melanocytes facilitates identification of melanoma susceptibility genes. Genome Res. 2018;28:1621–35.
Visconti A, Duffy DL, Liu F, Zhu G, Wu W, Chen Y, et al. Genome-wide association study in 176,678 Europeans reveals genetic loci for tanning response to sun exposure. Nat Commun. 2018;9. https://doi.org/10.1038/s41467-018-04086-y.
Han J, Kraft P, Nan H, Guo Q, Chen C, Qureshi A, et al. A genome-wide association study identifies novel alleles associated with hair color and skin pigmentation. PLoS Genet. 2008;4:1–12.
Spichenok O, Budimlija ZM, Mitchell AA, Jenny A, Kovacevic L, Marjanovic D, et al. Prediction of eye and skin color in diverse populations using seven SNPs. Forensic Sci Int Genet. 2011;5:472–8. https://doi.org/10.1016/j.fsigen.2010.10.005.
Candille SI, Absher DM, Beleza S, Bauchet M, McEvoy B, Garrison NA, et al. Genome-wide association studies of quantitatively measured skin, hair, and eye pigmentation in four European populations. PLoS One. 2012;7(10):e48294.
Nan H, Kraft P, Qureshi AA, Guo Q, Chen C, Hankinson SE, et al. Genome-wide association study of tanning phenotype in a population of european ancestry. J Invest Dermatol. 2009;129:2250–7. https://doi.org/10.1038/jid.2009.62.
Stacey SN, Sulem P, Masson G, Gudjonsson SA, Thorleifsson G, Jakobsdottir M, et al. New common variants affecting susceptibility to basal cell carcinoma. Nat Genet. 2009;41:909–14.
Duffy DL, Zhao ZZ, Sturm RA, Hayward NK, Martin NG, Montgomery GW. Multiple pigmentation gene polymorphisms account for a substantial proportion of risk of cutaneous malignant melanoma. J Invest Dermatol. 2010;130:520–8. https://doi.org/10.1038/jid.2009.258.
Stokowski RP, Pant PVK, Dadd T, Fereday A, Hinds DA, Jarman C, et al. A Genomewide association study of skin pigmentation in a south Asian population. Am J Hum Genet. 2007;81:1119–32. https://doi.org/10.1086/522235.
Sulem P, Gudbjartsson DF, Stacey SN, Helgason AS, Rafnar T, Magnusson KP, et al. Genetic determinants of hair, eye and skin pigmentation in Europeans. Nat Genet. 2007;39:1443–52.
Roadmap Epigenomics Consortium, Kundaje A, Meuleman W, Ernst J, Bilenky M, Yen A, et al. Integrative analysis of 111 reference human epigenomes. Nature. 2015;518:317–29.
Makoff AJ, Flomen RH. Detailed analysis of 15q11-q14 sequence corrects errors and gaps in the public access sequence to fully reveal large segmental duplications at breakpoints for Prader-Willi, Angelman, and inv dup (15) syndromes. Genome Biol. 2007;8(6):R114.
Lao O, De Gruijter JM, Van Duijn K, Navarro A, Kayser M. Signatures of positive selection in genes associated with human skin pigmentation as revealed from analyses of single nucleotide polymorphisms. Ann Hum Genet. 2007;71:354–69.
Akbari A, Vitti JJ, Iranmehr A, Bakhtiari M, Sabeti PC, Mirarab S, et al. Identifying the favored mutation in a positive selective sweep. Nat Methods. 2018. https://doi.org/10.1038/nmeth.4606.
Maroñas O, Phillips C, Söchtig J, Gomez-Tato A, Cruz R, Alvarez-Dios J, et al. Development of a forensic skin colour predictive test. Forensic Sci Int Genet. 2014;13:34–44.
Eiberg H, Troelsen J, Nielsen M, Mikkelsen A, Mengel-From J, Kjaer KW, et al. Blue eye color in humans may be caused by a perfectly associated founder mutation in a regulatory element located within the HERC2 gene inhibiting OCA2 expression. Hum Genet. 2008;123:177–87.
Visser M, Kayser M, Palstra RJ. HERC2 rs12913832 modulates human pigmentation by attenuating chromatin-loop formation between a long-range enhancer and the OCA2 promoter. Genome Res. 2012;22:446–55.
Liu F, van Duijn K, Vingerling JR, Hofman A, Uitterlinden AG, Janssens ACJW, et al. Eye color and the prediction of complex phenotypes from genotypes. Curr Biol. 2009;19:192–3.
Liu F, Wollstein A, Hysi PG, Ankra-Badu GA, Spector TD, Park D, et al. Digital quantification of human eye color highlights genetic association of three new loci. PLoS Genet. 2010;6:34.
Sturm RA, Duffy DL, Zhao ZZ, Leite FPN, Stark MS, Hayward NK, et al. A single SNP in an evolutionary conserved region within intron 86 of the HERC2 gene determines human blue-brown eye color. Am J Hum Genet. 2008;82:424–31.
Edwards M, Cha D, Krithika S, Johnson M, Cook G, Parra EJ. Iris pigmentation as a quantitative trait: variation in populations of European, east Asian and south Asian ancestry and association with candidate gene polymorphisms. Pigment Cell Melanoma Res. 2016;29:141–62.
Branicki W, Brudnik U, Wojas-Pelc A. Interactions between HERC2, OCA2 and MC1R may influence human pigmentation phenotype. Ann Hum Genet. 2009;73:160–70.
Eriksson N, Macpherson JM, Tung JY, Hon LS, Naughton B, Saxonov S, et al. Web-based, participant-driven studies yield novel genetic associations for common traits. PLoS Genet. 2010;6:1–20.
Liu F, Visser M, Duffy DL, Hysi PG, Jacobs LC, Lao O, et al. Genetics of skin color variation in Europeans: genome-wide association studies with functional follow-up. Hum Genet. 2015;134:823–35.
Walsh S, Chaitanya L, Breslin K, Muralidharan C, Bronikowska A, Pospiech E, et al. Global skin colour prediction from DNA. Hum Genet. 2017;136:865–6.
Bordogna W, Hudson JD, Buddle J, Bennett DC, Beach DH, Carnero A. EMX homeobox genes regulate microphthalmia and alter melanocyte biology. Exp Cell Res. 2005;311:27–38.
Eaton K, Edwards M, Krithika S, Cook G, Norton H, Parra EJ. Association study confirms the role of two OCA2 polymorphisms in normal skin pigmentation variation in east Asian populations. Am J Hum Biol. 2015;27:520–5.
Rawofi L, Edwards M, Krithika S, Le P, Cha D, Yang Z, et al. Genome-wide association study of pigmentary traits (skin and iris color) in individuals of east Asian ancestry. PeerJ. 2017;5:e3951. https://doi.org/10.7717/peerj.3951.
Sulem P, Gudbjartsson DF, Stacey SN, Helgason AS, Rafnar T, Jakobsdottir M, et al. Two newly identified genetic determinants of pigmentation in Europeans. Nat Genet. 2008;7:835–7.
Donnelly MP, Paschou P, Grigorenko E, Gurwitz D, Barta C, Lu RB, et al. A global view of the OCA2-HERC2 region and pigmentation. Hum Genet. 2012;131:683–96.
Delaneau O, Zagury JF, Marchini J. Improved whole-chromosome phasing for disease and population genetic studies. Nat Methods. 2013;10:5–6. https://doi.org/10.1038/nmeth.2307.
Durbin R. Efficient haplotype matching and storage using the positional burrows-wheeler transform (PBWT). Bioinformatics. 2014;30:1266–72.
Marchini J, Howie B. Genotype imputation for genome-wide association studies. Nat Rev Genet. 2010;11:499–511. https://doi.org/10.1038/nrg2796.
Kang HM. EPACTS (efficient and parallelizable association container toolbox). 2016. http://genome.sph.umich.edu/wiki/EPACTS.
Han B, Eskin E. Random-effects model aimed at discovering associations in meta-analysis of genome-wide association studies. Am J Hum Genet. 2011;88:586–98. https://doi.org/10.1016/j.ajhg.2011.04.014.
Han B, Eskin E. Interpreting meta-analyses of genome-wide association studies. PLoS Genet. 2012;8(3):e1002555.
Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–5.
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–75.
Das S, Forer L, Schönherr S, Sidore C, Locke AE, Kwong A, et al. Next-generation genotype imputation service and methods. Nat Genet. 2016;48:1284–7.
Wolpin BM, Rizzato C, Kraft P, Kooperberg C, Petersen GM, Wang Z, et al. Genome-wide association study identifies multiple susceptibility loci for pancreatic cancer. Nat Genet. 2014;46:994–1000. https://doi.org/10.1038/ng.3052.
Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21.
Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:323.
Stegle O, Parts L, Durbin R, Winn J. A bayesian framework to account for complex non-genetic factors in gene expression levels greatly increases power in eQTL studies. PLoS Comput Biol. 2010;6:1–11.
Ongen H, Buil A, Brown AA, Dermitzakis ET, Delaneau O. Fast and efficient QTL mapper for thousands of molecular phenotypes. Bioinformatics. 2016;32:1479–85.
Dayem Ullah AZ, Lemoine NR, Chelala C. SNPnexus: a web server for functional annotation of novel and publicly known genetic variants (2012 update). Nucleic Acids Res. 2012;40:65–70.
Ward LD, Kellis M. HaploReg v4: systematic mining of putative causal variants, cell types, regulators and target genes for human complex traits and disease. Nucleic Acids Res. 2016;44:D877–81.
Boyle AP, Hong EL, Hariharan M, Cheng Y, Schaub MA, Kasowski M, et al. Annotation of functional variation in personal genomes using RegulomeDB. Genome Res. 2012;22:1790–7.
Acknowledgements
The authors would like to acknowledge all the individuals who participated in this study. We thank Dr. Isabel Inês Araújo and the administrative staff at the University of Cape Verde for their support. This work utilized the computational resources of the National Institutes of Health high-performance computational capabilities Biowulf cluster (http://hpc.nih.gov). We acknowledge contributions to human melanocyte genotyping from The National Cancer Institute Cancer Genomics Research Laboratory (CGR) and to RNA sequencing from the National Institutes of Health Intramural Sequencing Center (NISC), and the National Cancer Institute Center for Cancer Research Sequencing Facility (CCR-SF) and the Yale University Skin SPORE Specimen Resource Core.
Funding
The genotyping of the Cuban sample was supported by the Ministry of Public Health, Cuba, and The Cuban-Danish collaborating project on Case-Controls Study for Bipolar Disorder and Schizophrenia. EJP received funding from the Natural Sciences and Engineering Research Council of Canada (NSERC Discovery Grant). Computations were performed on the GPC supercomputer at the SciNet HPC Consortium, Canada. SciNet is funded by: the Canada Foundation for Innovation under the auspices of Compute Canada; the Government of Ontario; Ontario Research Fund-Research Excellence; and the University of Toronto. FLD is supported by the National Council for Science and Technology (CONACYT) in Mexico. SB is currently supported by the Medical Research Council (grant number MR/M01987X/1). NH-P was funded by a fellowship (grant number FI16/00136) from the Instituto de Salud Carlos III (ISCIII) (NH-P) and co-funded by the European Social Funds from the European Union (ESF) “ESF invests in your future”. MP-Y was funded by the Ramón y Cajal Program (fellowship number RYC-2015-17205) by the Spanish Ministry of Economy, Industry and Competitiveness, and by ISCIII through AES and EC within AAL framework and the SysPharmPedia (award number AC15/00015), awarded from the ERACoSysMed 1st Joint Transnational Call from the European Union under the Horizon 2020. ST was supported by National Institutes of Health (grant numbers 1R01DK104339–0, 1R01GM113657–01) and the National Science Foundation (grant number BCS-1317217). EGB was funded by the Sandler Family Foundation; the American Asthma Foundation; the Robert Wood Johnson Foundation Amos Medical Faculty Development Program; Harry Wm. and Diana V. Hind Distinguished Professor in Pharmaceutical Sciences II; National Institutes of Health (grant numbers 1R01HL117004, R01Hl128439); National Institute of Health and Environmental Health Sciences (grant numbers R01ES015794, R21ES24844); the National Institute on Minority Health and Health Disparities (grant numbers 1P60MD006902, U54MD009523, 1R01MD010443) and the Tobacco-Related Disease Research Program under (award number 24RT-0025). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. KB, JC, TZ, and MK are supported by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics, National Cancer Institute, US National Institutes of Health. WP and SL are supported by the Intramural Research Program of the National Human Genome Research Institute. CAF-L was funded in part by the French “Agence Nationale pour la Recherche” grant METHIS (grant number ANR-15-CE32–0009-01).
Author information
Authors and Affiliations
Contributions
FLD: Drafting of manuscript/Analysis and interpretation of data, NH-P, SF, TZ, JC, MAK, SKL, PL, ME, CAF-L, CE, SH, DH, EJG-C, LCM-P, JG, OM, EGB, HLN, WJP: Acquisition of data/Analysis and interpretation of data. KMB, ST, MP-Y, SB, BMT: Analysis and interpretation of data/Critical revision. EJP: Study conception and design/Analysis and interpretation of data/Critical revision. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All methods were performed in accordance with the relevant guidelines and regulations for human subject research. The Cuba study was conducted following the ethical principles for medical research, and was approved by the Research Ethics Committee of the Cuban Centre of Medical Genetics at the Medical University of Havana, Cuba. Each participant gave a written informed consent prior to sample collection. The Cape Verde study was approved by the Human Subjects Committees of all participant institutions and by the National Ethical Committee for Health Research of Cape Verde, and written informed consent was obtained from all participants. The SAGE II and GALA II studies were approved by the institutional review boards of the University of California San Francisco and all participant centres. Written informed consent was obtained from all participants or from their surrogates for participants under 18 years old.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Figure S1. Distribution of M-index values in the Cuban sample. Figure S2. Manhattan plot depicting the results of the GWAS for the Cuban sample. Figure S3. QQ plot from the initial GWAS for the Cuban sample. Figures S4-S7. Correlations of African individual proportions and melanin index in the different samples. Figures S8-S9. QQ plots from the initial and conditional meta-analyses. Figures S10-S17. LD (r2 and D’) among the genome-wide significant SNPs in the OCA2/HERC2/APBA2 region of chromosome 15 in the Cuba and Cape Verde samples, and in the ASW and PUR populations of the 1KGP. Figures S18-S27. Regional plots of the OCA2/HERC2/APBA2 top markers on the region of chromosome 15. (DOCX 11908 kb)
Additional file 2:
Table S1. Top genome-wide significant SNPs in meta-analysis and on each independent study. Table S2. Top genome-wide significant SNPs in conditional meta-analysis and on each independent study. Table S3. Association tests conditioning for each of the lead signals in the OCA/HERC2/APBA2 region of Chromosome 15, for the Cape Verde and Cuba samples, separately. Tables S4-S5. Omnibus tests in the Cape Verde and Cuba samples separately, based on haplotypes from our lead signals (rs1448484, rs1667392, rs36194177, rs2636060). Table S6. Follow-up results of all genome-wide signals identified from our meta-analysis in an East African sample. Table S7. Follow-up of tanning response signals in our meta-analysis. Table S8. Follow-up of skin pigmentation signals identified in a recent GWAS in a large Latin American sample in our meta-analysis. (DOCX 75 kb)
Additional file 3:
The first sheet (‘Cuba’) includes the results obtained from the GWAS conducted in the Cuban sample, the second sheet (‘Meta’) includes the initial meta-analysis results, and the third sheet (‘Meta-cond’) includes the conditional meta-analysis results. (XLSX 843 kb)
Additional file 4:
Text S1 Supplementary information on the methods followed for the datasets included in the meta-analysis (Cape Verde, SAGE II, GALA II). (DOCX 28 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Lona-Durazo, F., Hernandez-Pacheco, N., Fan, S. et al. Meta-analysis of GWA studies provides new insights on the genetic architecture of skin pigmentation in recently admixed populations. BMC Genet 20, 59 (2019). https://doi.org/10.1186/s12863-019-0765-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12863-019-0765-5