Abstract
Background
Mainly based on evidence of success in adults, various medications are commonly used to prevent pediatric migraines. Topiramate has been approved for migraine prevention in children as young as 12 years of age. In this meta-analysis, we aimed to assess the currently published data pertaining to the efficacy of topiramate for migraine prevention in patients less than 18 years of age.
Methods
We searched PubMed/Medline, Embase and the Cochrane Library (from inception to April 2017) for randomized controlled trials (RCTs) published in English. Two independent investigators performed data extraction and quality evaluation using the Cochrane Collaboration’s tool. The data extracted were analyzed by Review Manager 5.3 software.
Results
A total of four RCTs matching the inclusion criteria were included, with an aggregate of 465 patients. Of these patients, 329 were included in the topiramate group, and 136 were included in the placebo group. This meta-analysis revealed that compared with placebo, topiramate failed to decrease the number of patients experiencing a ≥ 50% relative reduction in headache frequency (n = 465, RR = 1.26, 95% CI = [0.94,1.67], Z = 1.55, P = 0.12) or the number of headache days (n = 465, MD = −0.77, 95% CI = [−2.31,0.76], Z = 0.99, P = 0.32) but did reduce PedMIDAS scores (n = 205, MD = −9.02, 95% CI = [−17.34, −0.70], Z = 2.13, P = 0.03). Higher rates of side effects and adverse events in the topiramate group than in the placebo group were observed in the included trials.
Conclusions
Topiramate may not achieve a more effective clinical trial endpoint than placebo in the prevention of migraines in patients less than 18 years of age, and topiramate may lead to more side effects or adverse events in the included patients.
Similar content being viewed by others
Background
Migraine is the most common cause of headache in pediatric neurology outpatient clinics, and it has been recognized as one of the most prevalent neurological disorder in children and adolescents worldwide, affecting 5–10% of the pediatric population in multiple areas of life. Because patients miss school and social activities, migraines can impair the development of friendships that are vital to social development and self-esteem and may destroy family harmony [1, 2]. The mean age of onset of migraine is 7.2 years in boys and 10.9 years in girls [3], and the prevalence of migraine increases with age, as demonstrated by clinical studies. The diagnostic criteria for migraine headaches have developed over time; modern migraine classification includes frequency as a criterion, with episodic headaches occurring up to 14 days per month, and chronic migraine is defined as the persistence of headache without aura for at least 15 days per month and for at least 3 consecutive months without medication overuse (ICHD-II) [4]. Because of the diversity of symptoms, the diagnosis of migraines in children and adolescents needs to be refined even further. Due to the harm caused by migraines, reducing the number of migraine attacks to the greatest extent possible should be a priority.
At present, a variety of prophylactic therapy options are available to reduce the frequency or severity of headaches [5]. Topiramate is an antiepileptic drug with positive efficacy and safety for older children and adults with epilepsy [6], and it has been approved for migraine prevention in adults in Europe since 2003 and in the United States since 2004 [7]. The exact mechanism of topiramate in the treatment of migraine is unknown, although it may be associated with the influence of topiramate on pain transmission in the trigeminocervical complex and the third-order neurons in the ventroposteromedial thalamus [8]. Several case series and open-label trials [9,10,11,12,13] have shown that topiramate served as a preventive treatment for pediatric migraines, while the research of Scott W [14] indicated that there were no significant differences between topiramate and placebo in the prevention of pediatric migraine. Hence, in the present study, we performed a meta-analysis of randomized controlled trials (RCTs) to evaluate the efficacy of topiramate for the prevention of migraine in patients less than 18 years of age.
Methods
Protocol registration
The protocol registration number was CRD42017062287 (http://www.crd.york.ac.uk/Prospero).
Data sources and search strategies
We searched using the following databases: PubMed/Medline, Embase and the Cochrane Library (inception to April 2017) to retrieve the RCTs of topiramate in migraine prevention for patients less than 18 years of age. The following search terms were used in combination: (“topiramate” OR “topamax”) AND (“migraine disorders” OR “migraine” OR “migraineur” OR “migraineurs” OR “migrain” OR “sick headache”) AND (“pediatric” OR “adolescent” OR “adolescence” OR “child” OR “children” OR “childhood” OR “teen” OR “youth”). The references of eligible studies, relevant systematic reviews, and meta-analysis were also manually retrieved. The publication language was restricted to English.
Study selection
The automatically retrieved studies were evaluated by two independent investigators and included in the meta-analysis based on the criteria presented below. The reviewers resolved any differences by consensus. The investigators selected the retrieved studies that matched the inclusion and exclusion criteria.
Inclusion criteria
Studies were included in the meta-analysis if they fulfilled the following criteria: (1) the study was a trial comparing topiramate with placebo in migraine patients, (2) the study had similar diagnostic criteria for migraine or definition of migraine, (3) the age of the participants was less than 18 years, (4) the study was a clinical RCT, (5) the intent-to-treat population numbers in the topiramate and placebo groups were provided, and (6) the number of participants showing ≥50% reduction in headache frequency, baseline and follow-up data of headache days or PedMIDAS scores were available.
Exclusion criteria
Studies were excluded according to the following exclusion criteria: (1) the study was not a RCT but a review, case report, letter, editorial or other type of publication not describing original research, (2) the full text was not available, (3) the study did not afford extractable outcomes, (4) the control group of the trial did not contain placebo (for example, the trial only used propranolol or sodium valproate as a control), and (5) the trial involved adults and children, but the characteristics or outcomes of the pediatric subgroup were unavailable or unextractable.
Risk of bias in individual studies
The methodological quality of RCTs was evaluated according to the risk of bias tool described in the Cochrane Handbook for Systematic Reviews of Interventions [15]. Seven quality elements that contain random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and baseline balance bias were assessed. Study selection, data extraction and risk bias assessment were conducted by two researchers (Kai Le and Dafan Yu) independently; in case of discrepancies consensus was reached by discussion with a third party (Yijing Guo).
Data extraction
Our primary outcome was a relative reduction in the number of headache days of 50% or more in the comparison of the 28-day baseline period with the last 28 days. Secondary outcomes included headache days and PedMIDAS scores. The PedMIDAS score, which is used to ascertain a change in headache-related disability [16] between the beginning and the end of the trial and the decrease in the number of headache days from the 28-day baseline period to the final 28-day period of treatment were recorded. The main information, including the numbers of participants in the topiramate and placebo groups, the diagnostic tool, the dose and duration of topiramate, the numbers of patients experiencing a ≥ 50% relative reduction in headache frequency, the mean headache days per 28-day period and the mean PedMIDAS score in both groups, was extracted. Additional information was also abstracted, such as publication year, first author, age and sex. Side effects and adverse events after drug administration were also recorded if they occurred. The two investigators (Kai Le and Dafan Yu) extracted all the data independently. If there was any disagreement between the two reviewers, they resolved it by discussion and consensus, with a third party participating if necessary.
Statistical analysis
The meta-analysis was conducted using Review Manager 5.3 software (Cochrane Collaboration, Copenhagen, Denmark). Continuous data are presented as the mean difference (MD) with a 95% confidence interval (CI) and inverse variance (IV). Dichotomous outcomes were analyzed by pooled risk ratio (RR) with a 95% CI to present effect estimate and Mantel-Haenszel test. The heterogeneity among eligible trials was quantified using a chi-squared-based Q-statistic test (P < 0.1, suggesting the existence of heterogeneity). An I 2 statistic was alsoused to quantify the inconsistency across studies, with I 2 > 50% considered statistically significant. When there was no statistically significant heterogeneity, we used a fixed-effects model for pooling the results; otherwise, a random-effects model was used. A 2-sided P value <0.05 was taken to indicate statistical significance for 1 comparison group over the other. The results of the meta-analysis were visualized using forest plots. Visual inspection of funnel plots was used to assess possible publication bias if more than 10 trials were identified that reported on the same outcome [17].
Results
Search results
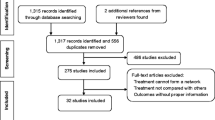
A total of 541 articles were identified from among 56 listed in PubMed/Medline, 429 in Embase and 56 in the Cochrane Library. After excluding 82 duplicates, 459 potentially eligible articles were selected. Of these articles, 401 were excluded through titles and abstracts, leaving 58 articles for further evaluation. The reasons for exclusion during full-text review were “studies involved adults only” (n = 15), “studies included adults and children” (n = 14), “conference abstract” (n = 2), “editorial” (n = 3), “not controlled” (n = 6), “no placebo” (n = 9), “insufficient data” (n = 4) and “protocol” (n = 1). Finally, 4 prospective RCTs [14, 18,19,20] were included in our meta-analysis. The research process is shown in Fig. 1.
Characteristics of the included RCTs
The 4 included studies were published between 2005 and 2017. Study sample sizes ranged from 42 to 163, with a total of 465 randomized patients, including 329 patients in the topiramate group and 136 in the placebo group, and the age of the participants varied from 8 to 17 years old. To diagnose a migraine, one trial [20] employed the International Headache Society (IHS) diagnostic criteria for pediatric migraine and 3 trials [14, 18, 19] used the International Classification of Headache Disorders, 2nd Edition (ICHD-II) [21]. One trial [18] used 2 doses of topiramate versus placebo. Another trial [14] used amitriptyline and topiramate as the two treatment arms, and we extracted the results of topiramate versus placebo. The duration of the included trials consisted of titration and maintenance periods: 2 trials [18, 19] lasted 16 weeks, one trial [20] lasted 20 weeks, and one trial [14] lasted 24 weeks. The detailed characteristics of the studies included in the meta-analysis are listed in Table 1.
Risk of bias of the included trials
All RCTs described the procedure of randomization and blinded participants and researchers, and all trials reported allocation concealment and blinding of outcome assessment. All outcome data were complete. Detailed information is shown in Fig. 2.
Meta-analysis
Primary outcome
As shown in Table 2, all 4 included trials investigated the effects of topiramate on migraine prevention via the numbers of patients experiencing a ≥ 50% relative reduction in headache frequency. The results of our meta-analysis show that, there were no significant differences between the topiramate and placebo groups in terms of the numbers of patients experiencing a ≥ 50% relative reduction in headache frequency (n = 465, RR = 1.26, 95% CI = [0.94, 1.67], Z = 1.55, P = 0.12) (Fig. 3). The evidence collected in our meta-analysis shows heterogeneity (I 2 = 59%). Analysis was performed by a random-effects model. The z-test result for overall effects showed no statistical significance (P = 0.12).
Secondary outcomes
All 4 trials included in our meta-analysis reported mean headache days from the 28-day baseline period to the final 28-day period of treatment, and 2 trials [14, 19] presented mean PedMIDAS scores (Table 2). We found no significant difference in mean headache days between the topiramate and placebo groups (n = 465, MD = −0.77, 95% CI = [−2.31, 0.76], Z = 0.99, P = 0.32) (Fig. 4). The evidence collected in our meta-analysis shows considerable heterogeneity (I 2 = 70%). Analysis was performed using a random-effects model. The z-test result for overall effects showed no statistical significance (P = 0.32). We did find significant differences in the mean PedMIDAS score between the two groups (n = 205, MD = −9.02, 95% CI = [−17.34, −0.70], Z = 2.13, P = 0.03) (Fig. 5). The evidence collected in our meta-analysis shows heterogeneity (I 2 = 52%). Analysis was performed suing a random-effects model. The z-test result for overall effects was statistically significant (P = 0.03).
Side effects and adverse events
All included trials reported side effects or adverse events such as paresthesia, weight decrease, anorexia, fever, fatigue, upper respiratory tract infection, somnolence, allergy, and traumatic liver injury. The overall incidence of most adverse events was higher in the topiramate group than in the placebo group, with ten of these events (including suicide attempts and other disabling events) occurring only in the topiramate group. Detailed side effects and adverse events and their frequency in both groups in the included studies are listed in Table 3. We also performed a meta-analysis of each side effect or adverse event that was reported in at least two RCTs. As shown in Fig. 6, there was a significant increase in paresthesia (Fig. 6a, n = 483, RR = 5.04, 95% CI = [2.13, 11.94]; Z = 3.68, P = 0.0002) and weight decrease (Fig. 6b, n = 380, RR = 4.38, 95% CI = [1.92, 10.01], Z = 3.51, P = 0.0005) in the topiramate group. The evidence collected in our meta-analysis shows no obvious heterogeneity (I 2 = 0%).Analysis was performed using a fixed-effects model.
Forest plot of comparison: Side effects/adverse events (a-m, respectively, represent paresthesia,weight decrease, abdominal pain, anorexia, fatigue, injury, upper respiratory tract infection, dizziness, fever, nausea, pharyngitis, sinusitis and somnolence) of topiramate versus placebo(*There was a significant difference between topiramate and placebo groups)
Publication bias
Since our meta-analysis included only four studies, a linear regression test of funnel plot asymmetry (Egger’s test) could not be performed.
Discussion
This meta-analysis examined the efficacy of topiramate in comparison with placebo for the prevention of migraines in patients less than 18 years of age. The IHS guidelines for conducting clinical trials indicate that a clinically meaningful end point in a migraine prevention trial is usually defined by a reduction in the total number of headache attacks in a 28-day period or the proportion of patients with a greater than 50% relative reduction in headache frequency [22].
Topiramate is a first-line option for the treatment of migraines in adults, and in March 2014, the U.S. Food and Drug Administration (FDA) approved topiramate for migraine prevention in the population aged 12 to 17 [23]. Moreover, this is the first and only medication currently approved for use in migraine patients 12 years and over. Nevertheless, neither the primary outcome of proportion of patients with a greater than 50% reduction in headache frequency nor the secondary outcome of reduced mean headache days in a 28-day period showed topiramate as more efficacious than placebo in our meta-analysis of four RCTs. According to the definition [22], topiramate showed no statistically significant benefit over placebo in reducing the number of headache days over the treatment period. In fact, the 50% response rate of the topiramate group in 2 trials [14, 20] was not statistically significant compared with the placebo group, and in another trial [18] a similar result was presented for the 50 mg/day topiramate treatment group. The finding conflicts with the outcomes of previous case series and open-label trials. There are at least three possible explanations for this finding. (1) Children tend to have a high placebo response rate, with younger patients in clinical trials demonstrating a greater tendency to respond to placebo. This age-dependent placebo response has ranged from 30% to 70% in migraine studies in general [24,25,26,27]. The outcome of our study shows that the average number of patients experiencing a ≥ 50% relative reduction in headache frequency in the placebo group is 50.74% (69/136), which is higher than the rates reported in previous studies of topiramate preventing adult migraine (0–34.2%) [28,29,30,31]. Rothner et al. [32] suggested explanations for the higher placebo response rate in clinical trials with children and adolescents, such as the fact that they could not take medication while at school; “good doctor” effects; and the fact that if their symptoms relieved spontaneously, children and adolescents were more likely than adults to believe that they were receiving a drug that had a definite effect on headache. We speculate that this phenomenon is associated with at least the following factors: 1. Different psychological and neurobiological mechanisms exist in pediatric patients compared with adults. There are at least four psychological mechanisms associated with the placebo response: expectation, conditioning, therapeutic relationship and empowerment [33]; psychological mechanisms, especially the conditioning and expectation may guide people’s behavior. The differential course of the maturation of different neurotransmitter systems may explain the differences. 2. The characteristics of migraine attacks are different [34]: migraine headaches in children and adolescents are often bilateral and may be of shorter duration than in adults. 3. Children/adolescents and adults have significantly different cognitive levels. The pain sensation is a highly subjective experience that is influenced by cognitive factors, and placebo analgesia is one of the most striking examples of cognitive regulation of pain [35, 36]. In addition, the lack of pediatric research and the shortage of experience in experimental design may lead to different outcomes. In short, the topic of the difference about placebo response between children/adolescents and adults deserves further discussion. (2) The minimum age at which topiramate was approved for treatment of migraine was 12 years old, but the minimum age of patients in the included trials was 8 years. It is often difficult to calculate the attacks of headache in younger children accurately, and the guardians generally interpret the attacks indirectly from the child’s activity level [19]. (3) Our included patients included those with either episodic or chronic migraine [14], which may influence the results of our meta- analysis.
The second finding of our meta-analysis is that topiramate decreased PedMIDAS scores in migraine patients. PedMIDAS is often used to measure disability related to school absences and functioning, home functioning, and social absences and functioning [16]. This finding, which contradicts our first finding, may indicate that headache-related disability is alleviated by topiramate. However, mean PedMIDAS scores in both the topiramate group and the placebo group decreased between baseline and endpoint, and the fact that only two trials [14, 19] used this tool as a trial assessment may be the cause of the heterogeneity.
As with all antiepileptic drugs, topiramate has many potential side effects or adverse events, some of which may be serious and life-threatening [37]. The rate of adverse events in patients treated with topiramate was higher than that with placebo in our included trials. It has been reported that metabolic acidosis, renal calculi and nervous system effects, such as fatigue or somnolence, paresthesia, dizziness and cognitive disorder or aphasia, occurred in adults and pediatric patients taking topiramate in previous trials. Other adverse events, such as changes in visual acuity, including visual field deficits, acute myopia and secondary closed angle glaucoma, have also been reported. In addition, topiramate (100 mg/day) was related to modest increases in psychomotor reaction times [38]. Another more serious problem is the potential for suicidal behavior and ideation that has been observed in people taking antiepileptic drugs, including topiramate [39]. Thus, while the pathomechanism of migraine is not completely understood, the choice of medication for personalized therapy tailored to each patient needs to be made cautiously [40].
Some limitations in our meta-analysis must be acknowledged. First, because our analysis was limited to articles in the English language literature, we may have omitted some evidence. The secondary limitation is related to the data that we acquired from the four included trials. Three of the trials reported the baseline and follow-up data [14, 18, 19], and one reported baseline and change data [20]. We combined the follow-up and change data according to the research of da Costa, B. R [41]. In addition, one trial compared more than 1 dose [18]; it is likely that dose-finding pharmacologic studies are underrepresented and that additional unpublished industry trials exist. These situations might have resulted in ecological bias. Third, our data had obvious heterogeneity, and none of the variables we abstracted explained this variation. Because we only included four trials and because only three measurements were used in our study, therefore, our findings should be interpreted with caution. The variability in the selection criteria for RCTs and sample size, along with the incomplete reporting of intervention intensity, may also be limitations.
Conclusions
This is the first meta-analysis of topiramate for migraine prevention in patients less than 18 years of age. We found that topiramate did not achieve a more effective clinical trial endpoint than placebo in the prevention of migraine in patients less than 18 years of age, and topiramate was associated with more adverse events in the included patients. It is possible that a high placebo response rate can be beneficial for children and adolescents with migraine and that drugs used to prevent pediatric migraine might be reconsidered. Because there was a significant placebo response, more placebo-controlled trials in the younger migraine population less than 12 years of age are needed.
References
Split W, Neuman W (1999) Epidemiology of migraine among students from randomly selected secondary schools in Lodz. Headache 39(7):494–501
Kacperski J (2015) Prophylaxis of migraine in children and adolescents. Paediatr Drugs 17(3):217–226. doi:10.1007/s40272-015-0125-5
Lewis D, Ashwal S, Hershey A, Hirtz D, Yonker M, Silberstein S (2004) Practice parameter: pharmacological treatment of migraine headache in children and adolescents: report of the American Academy of Neurology quality standards subcommittee and the practice Committee of the Child Neurology Society. Neurology 63(12):2215–2224
Lewis DW, Avener M, Gozzo Y (2005) Pediatric migraine, part 1: update on classification, diagnosis, and the evaluation. Headache Pain Diagn Challenges Curr Ther 16(2):81–88
Toldo I, De Carlo D, Bolzonella B, Sartori S, Battistella PA (2012) The pharmacological treatment of migraine in children and adolescents: an overview. Expert Rev Neurother 12(9):1133–1142. doi:10.1586/ern.12.104
French JA, Kanner AM, Bautista J, Abou-Khalil B, Browne T, Harden CL, Theodore WH, Bazil C, Stern J, Schachter SC et al (2004) Efficacy and tolerability of the new antiepileptic drugs II: treatment of refractory epilepsy: report of the therapeutics and technology assessment subcommittee and quality standards Subcommittee of the American Academy of neurology and the American Epilepsy Society. Neurology 62(8):1261–1273
Brandes JL, Saper JR, Diamond M, Couch JR, Lewis DW, Schmitt J, Neto W, Schwabe S, Jacobs D (2004) Topiramate for migraine prevention: a randomized controlled trial. JAMA 291(8):965–973. doi:10.1001/jama.291.8.965
Hoffmann J, Akerman S, Goadsby PJ (2014) Efficacy and mechanism of anticonvulsant drugs in migraine. Expert Rev Clin Pharmacol 7(2):191–201. doi:10.1586/17512433.2014.885835
Campistol J, Campos J, Casas C, Herranz JL (2005) Topiramate in the prophylactic treatment of migraine in children. J Child Neurol 20(3):251–253
Anand KS, Dhikav V, Aggarwal J (2012) Topiramate for migraine prophylaxis. Indian Pediatr 49(4):329–330. doi:10.1007/s13312-012-0040-6
Abbaskhanian ASHREAMS (2012) Effective dose of topiramate in pediatric migraine prophylaxis. In J Pediatr Neurosci 7:171–174
Fallah R, Divanizadeh MS, Karimi M, Mirouliaei M, Shamszadeh A (2013) Topiramate and propranolol for prophylaxis of migraine. Indian J Pediatr 80(11):920–924. doi:10.1007/s12098-013-0976-0
Hershey AD, Powers SW, Vockell ALB, LeCates S, Kabbouche M (2002) Effectiveness of topiramate in the prevention of childhood headaches. Headache 42(8):810–818. doi:10.1046/j.1526-4610.2002.02185.x
Powers SW, Coffey CS, Chamberlin LA, Ecklund DJ, Klingner EA, Yankey JW, Korbee LL, Porter LL, Hershey AD (2017) Trial of Amitriptyline, Topiramate, and placebo for pediatric migraine. N Engl J Med 376(2):115–124. doi:10.1056/NEJMoa1610384
Higgins JP, Green S (2011) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (The Cochrane Collaboration).
Hershey AD, Powers SW, Vockell AL, LeCates S, Kabbouche MA, Maynard MK (2001) PedMIDAS: development of a questionnaire to assess disability of migraines in children. Neurology 57(11):2034–2039
Deeks JJ, Macaskill P, Irwig L (2005) The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J Clin Epidemiol 58(9):882–893. doi:10.1016/j.jclinepi.2005.01.016
Lewis D, Winner P, Saper J, Ness S, Polverejan E, Wang S, Kurland CL, Nye J, Yuen E, Eerdekens M et al (2009) Randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of topiramate for migraine prevention in pediatric subjects 12 to 17 years of age. Pediatrics 123(3):924–934. doi:10.1542/peds.2008-0642
Lakshmi CVS, Singhi P, Malhi P, Ray M (2007) Topiramate in the prophylaxis of pediatric migraine: a double-blind placebo-controlled trial. J Child Neurol 22(7):829–835. doi:10.1177/0883073807304201
Winner P, Pearlman EM, Linder SL, Jordan DM, Fisher AC, Hulihan J (2005) Topiramate for migraine prevention in children: a randomized, double-blind, placebo-controlled trial. Headache 45(10):1304–1312. doi:10.1111/j.1526-4610.2005.00262.x
2014) The International Classification of Headache Disorders: 2nd edition. Cephalalgia : an international journal of headache, 24 Suppl 1:9–160
Tfelt-Hansen P, Pascual J, Ramadan N, Dahlof C, D'Amico D, Diener HC, Hansen JM, Lanteri-Minet M, Loder E, McCrory D et al (2012) Guidelines for controlled trials of drugs in migraine: third edition. A guide for investigators. Cephalalgia Int J Headache 32(1):6–38. doi:10.1177/0333102411417901
2014) FDA approves Topamax for migraine prevention in adolescents. J Pain Palliat Care Pharmacother, 28(2):191
Hershey AD (2010) Current approaches to the diagnosis and management of paediatric migraine. Lancet Neurol 9(2):190–204. doi:10.1016/s1474-4422(09)70303-5
Aaltonen K, Hamalainen M, Hoppu K (2000) Children’s response to placebo in migraine attacks. Cephalalgia Int J Headache 20:385
Bendtsen L, Mattsson P, Zwart JA, Lipton RB (2003) Placebo response in clinical randomized trials of analgesics in migraine. Cephalalgia An Int J Headache 23(7):487–490. doi:10.1046/j.1468-2982.2003.00528.x
Faria V, Linnman C, Lebel A, Borsook D (2014) Harnessing the placebo effect in pediatric migraine clinic. J Pediatr 165(4):659–665. doi:10.1016/j.jpeds.2014.06.040
Silberstein SD, Hulihan J, Karim MR, Wu SC, Jordan D, Karvois D, Kamin M (2006) Efficacy and tolerability of topiramate 200 mg/d in the prevention of migraine with/without aura in adults: a randomized, placebo-controlled, double-blind, 12-week pilot study. Clin Ther 28(7):1002–1011. doi:10.1016/j.clinthera.2006.07.003
Diener HC, Bussone G, Van Oene JC, Lahaye M, Schwalen S, Goadsby PJ (2007) Topiramate reduces headache days in chronic migraine: a randomized, double-blind, placebo-controlled study. Cephalalgia : an international journal of headache 27(7):814–823. doi:10.1111/j.1468-2982.2007.01326.x
Freitag FG, Forde G, Neto W, Wang DZ, Schmitt J, Wu SC, Hulihan J (2007) Analysis of pooled data from two pivotal controlled trials on the efficacy of topiramate in the prevention of migraine. J Am Osteopath Assoc 107(7):251–258
Gupta P, Singh S, Goyal V, Shukla G, Behari M (2007) Low-dose topiramate versus lamotrigine in migraine prophylaxis (the Lotolamp study). Headache 47(3):402–412. doi:10.1111/j.1526-4610.2006.00599.x
Rothner AD, Wasiewski W, Winner P, Lewis D, Stankowski J (2006) Zolmitriptan oral tablet in migraine treatment: high placebo responses in adolescents. Headache 46(1):101–109. doi:10.1111/j.1526-4610.2006.00313.x
Antonaci F, Chimento P, Diener HC, Sances G, Bono G (2007) Lessons from placebo effects in migraine treatment. J Headache Pain 8(1):63–66. doi:10.1007/s10194-007-0360-4
Diener HC, Schorn CF, Bingel U, Dodick DW (2008) The importance of placebo in headache research. Cephalalgia Int J Headache 28(10):1003–1011. doi:10.1111/j.1468-2982.2008.01660.x
Kupers R, Faymonville ME, Laureys S (2005) The cognitive modulation of pain: hypnosis- and placebo-induced analgesia. Prog Brain Res 150:251–269. doi:10.1016/s0079-6123(05)50019-0
Ploghaus A, Becerra L, Borras C, Borsook D (2003) Neural circuitry underlying pain modulation: expectation, hypnosis, placebo. Trends Cogn Sci 7(5):197–200
Oakley CB, Kossoff EH (2014) Migraine and epilepsy in the pediatric population. Curr Pain Headache Rep 18(3):402. doi:10.1007/s11916-013-0402-3
Pandina GJ, Ness S, Polverejan E, Yuen E, Eerdekens M, Bilder RM, Ford L (2010) Cognitive effects of topiramate in migraine patients aged 12 through 17 years. Pediatr Neurol 42(3):187–195. doi:10.1016/j.pediatrneurol.2009.10.001
Fantasia HC (2014) Migraine headache prophylaxis in adolescents. Nurs Women’s Health 18(5):420–424. doi:10.1111/1751-486X.12150
Tajti J, Szok D, Csáti A, Vécsei L (2016) Prophylactic drug treatment of migraine in children and adolescents: an update. Curr Pain Headache Rep 20(1):1–9. doi:10.1007/s11916-015-0536-6
da Costa BR, Nuesch E, Rutjes AW, Johnston BC, Reichenbach S, Trelle S, Guyatt GH, Juni P (2013) Combining follow-up and change data is valid in meta-analyses of continuous outcomes: a meta-epidemiological study. J Clin Epidemiol 66(8):847–855. doi:10.1016/j.jclinepi.2013.03.009
Acknowledgments
This study was supported by National Natural Science Foundation of China (NO. 81471187).
We also acknowledge all clinical investigators of the included studies and patients associated with these studies.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article.
Author information
Authors and Affiliations
Contributions
Kai Le, Yijing Guo participated in the whole design of this study. Kai Le, Yijing Guo and Dafan Yu performed the database search, data extraction and analysis. Kai Le, Yijing Guo, Dafan Yu and Jiamin Wang wrote the draft and revised the whole manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Le, K., Yu, D., Wang, J. et al. Is topiramate effective for migraine prevention in patients less than 18 years of age? A meta-analysis of randomized controlled trials. J Headache Pain 18, 69 (2017). https://doi.org/10.1186/s10194-017-0776-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s10194-017-0776-4