Abstract
Background
The pro-apoptotic Bcl-2 protein BAD initiated apoptosis in human cells and has been identified as a prognostic marker in non-small cell lung cancer (NSCLC). In this study, we aimed to explore the functions of BAD in NSCLC.
Methods
Overexpression of BAD was performed by transfecting different NSCLC cell lines with wild-type BAD. Cell proliferation, cell cycle, apoptosis, and invasion were characterized in vitro. Tumorigenicity was analyzed in vivo. Western blot was performed to determine the effects of BAD overexpression on the Bcl-2 family proteins and apoptosis-related proteins.
Results
Overexpression of BAD significantly inhibited cell proliferation in H1299, H292, and SPC-A1 but not in SK-MES-1 and H460 cell lines in vitro. BAD overexpression also reduced the tumorigenicity of H1299/SPC-A1 cell in vivo. However, no appreciable effects on cell cycle distribution and invasion were observed in all these cell lines. BAD overexpression also induced apoptosis in all cell types, in which process expression of mitochondrial cytochrom c (cyto-c) and caspase 3 were increased, whereas Bcl-xl, Bcl-2, Bax and caspase 8 expressions did not changed. These findings indicated that a mitochondrial pathway, in which process cyto-c was released from mitochondrial to activate caspase 3, was involved in BAD overexpression-mediated apoptosis.
Conclusions
Our data suggested that increased expression of BAD enhance apoptosis and has negative influence on cell proliferation and tumor growth in NSCLC. Bad is a new potential target for tumor interventions.
Similar content being viewed by others
Background
Lung cancer is the most frequent cancer worldwide with the highest cancer-related mortality [1]. The American Cancer Society estimates that in 2013, there would be 159,480 deaths from lung cancer and 228,190 new cases diagnosed. Of these, non-small cell lung cancer (NSCLC) account for ~ 85%. Currently therapeutic interventions have little influence on the prognosis of patients with NSCLC. The 5-year survival rates remain less 15% and recurrence rate still increased after surgery [2]. Mechanism that enable cancer cell to evade apoptosis may contribute to therapeutic resistance, which is a major challenge for prognostic improvement in NSCLC. Thus, anticancer agents triggered the cell undergoing apoptosis could improve response to treatment and clinical outcome. We recently reported that the BAD (Bcl-Xl/Bcl-2-associated death promoter homologue), as a pro-apoptotic Bcl-2 protein, shows decreased expression level, and plays an important role for predicting therapeutic response and survival in NSCLC [3].
BAD, as one of the “death-promoting” members of the Bcl-2 family [4], is activated through dephosphorylation responsible for apoptotic event, in contrast, inactivation promote cell survival through phosphorylation on several serine residues by upstream kinase, such as Akt, Raf, Pim-2, and PKA [3, 5]. It is largely assumed that BAD interact with pro-survival Bcl-2 family proteins, including Bcl-2 and Bcl-xl, to ablate their pro-survival function. This ablation allows activation of downstream, such as Bax and Bak,to induce cell to apoptosis [6, 7]. BAD protein has been reported to contribute to tumorigenesis and chemotherapy resistant. Ranger et al. found that BAD-deficient mice develop diffuse large B cell lymphoma [8]. BAD−/− mammary cancer cells are resistant to gefitinib-therapy in the study by Gilmore [9]. Moreover, BAD have been shown to be prognostic biomarkers for colon cancer, ovarian cancer, and breast cancer patients [10, 11]. Our previous studies also provided clinical evidence that loss of BAD is an independent and powerful predictor of adverse prognosis in NSCLC [3]. Therefore, in addition to regulating apoptosis, BAD might be involved in various cellular functions, such as proliferation and tumor growth in NSCLC.
In this study, our data provided experimental evidence that BAD could play functions as a tumor suppressor in NSCLC. Increased BAD expression has effects on proliferation of NSCLC cell lines and tumor growth in vivo. Meanwhile, BAD overexpression induced apoptosis in all cell types, in which process cytochrom c (cyto-c) and caspase 3 releases was involved.
Methods
Cell culture and animal models
The following cell lines were obtained from the Type Culture Collection of the Chinese Academy of Sciences and cultured according to recommendations: NCI-H1299 (metastatic NSCLC, H1299), NCI-H292 (mucoepidermoid adenocarcinoma cell, H292), NCI-H460 (large cell carcinoma cells, H460), SPC-A1 (pulmonary adenocarcninoma cells), and SK-MES-1 (squamous cell carcinoma cells, SK-MES). H1299/H292/H460/SPC-A1/SK-MES-BAD cells (NSCLC cell-BAD) were generated by transfecting those cell lines with wild-type BAD (Pdonr223/BAD, Neuron Biotech, Shanghai, China) as previously described [12]. H1299/H292/H460/SPC-A1/SK-MES-NC cells (NSCLC cell-NC) refer to the cell lines transfected with empty vector (pLOV. UBC. EGFP, Neuron Biotech, Shanghai, China). All cultured cells were maintained in a humidified 5% CO2 atmosphere at 37°C. Nude, 5-8-week-old athymic nude mice (BALB/c-nu/nu nude mice), half of which were female and half male were obtained from the Laboratory Animal Centre of Sichuan University. All mice were housed in laminar flow cabinets under specific, pathogen-free conditions with food and water provided ad libitum. All animal procedures listed in this article were performed in accordance with the Helsinki Convention for the use and care of animals, and approved by the Institutional Animal Care and Treatment Committee of Sichuan University.
Tissue protein extraction and western blot assay
Total protein was extracted from cultured cells and xenograft tumours using the whole protein extraction kit (KeyGEN, Nanjing, China), Protein concentration were measured using BCA Protein Assay Reagent (Thermo scientific, Rockford, USA). Equivalent amounts of protein from different samples were subjected to sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) using polyvinylidene fluoride (PVDF) membranes (Millipore, Billeraica, USA) to electro blot. The membranes were incubated overnight at 4°C with anti-BAD monoclonal antibody (#9292, 1;1000, Cell Signaling Technology, Beverly, USA), Bcl-xl (#24247, 1:1000, Signalway Antibody, Pearland, USA), Bcl-2 (#24246, 1:1000, Signalway Antibody), Bax (#24250, 1:1000, Signalway Antibody), Caspase 3 (#21420, 1:1000, Signalway Antibody), Caspase 8 (#21421, 1:1000, Signalway Antibody), cytochrome C (#21680, 1:1000, Signalway Antibody) and β-actin (#4970, 1:5000, Cell Signaling Technology). Target proteins were detected using the ChemiDoc XRS system (Bio-rad, Philadelphia, USA) by exposure to chemiluminescent HRP substrate (Millipore, Billerica, USA) and analyzed via Quantity One 1-D Analysis software (Bio-rad) [3, 13].
Cell proliferation assay
The effects of BAD on the proliferation of NSCLC cell lines were determined using Cell Counting kit-8 (CCK-8; Dojindo, Kumamoto, Japan) according to the manufacturer’s protocol. Briefly, cells were seeded in 96-well plates at a density of 2 × 103 per well. The plates were incubated at 37°C for 1, 2, 3, 4, 5 and 6 days. Then the optical density was measured at 450 nm to determine cell proliferation index. Results are presented as the mean ± SD based on at least three independent experiments.
Cell cycle analysis
Approximately 1 × 106 cells were harvested and washed 3 times with PBS and switch to media containing 0.05% fetal bovine serum (FBS). Then the cells were re-suspended in DNA staining solution [(PI, 50 μg/ml), RNase A (100 μg/ml)), and 0.1% (vol/vol) Triton X-100 in PBS) and incubated at room temperature for 30 min. The DNA content was determined by FACS Calibar Flow Cytometer (Becton-Dickinson, Franklin Lakes, USA). Data were analyzed using CellQuest and Modfit software.
Invasion assay
The invasion of NSCLC cell lines were measured using the BD BioCoat Tumor Invasion Assay System (BD Bioscience) according to the manufacture’s protocol. Cell suspensions containing 1 × 106 cells/ml were seeded onto the upper chamber with serum free media. DMEM containing 10% FBS, as a chemoattractant, was then added to the lower chamber. After 48 h of incubation, the invasive cells on the lower surface of the membrane were stained by dipping the inserts into staining solution for 20 min. The cells were measured by photographing the membrane using a microscope at five random views.
Apoptosis assessment
Approximately 5 x 105 cells were harvested, washed in PBS and incubated with 5 μl Annexin V-APC and 7AAD (KeyGen, Nanjing, China) at room temperature for 5 min. Fluorescence was measured using a FACS Calibar Flow Cytometer. The Annexin V-positive and 7AAD-negative cells were regarded as apoptotic.
The TUNEL (terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling) assay was performed to detect early stage of DNA fragmentation in apoptotic cells by using a commercial kit (In situ Cell Death Detection kit, TMR red, Roche Diagnostics Limited, Shanghai, China). Paraffin-embedded xenograft tumor sections were permeabilized in 0.1% Triton X-100, and then incubated with TUNEL reaction mixture containing TdT and TMR-dUTP. During this incubation step, TdT catalyzes the attachment of TMR-dUTP to free 3’OH ends in the DNA. Nuclei were counterstained with DAPI. Tissue sections were analyzed for apoptotic cells with localized TMR red under a fluorescence microscope. The apoptotic cell rate was determined according to the formula: (number of apoptotic cell with red staining/total number tumor cells with DAPI staining) × 100%.
Immunohistochemistry
IHC assay was performed on histological sections of formalin-fixed tumor xenografts as previously described [3, 14]. The primary antibody is proliferative marker ki-67 (1:100, Dako). The positive cell rate was measured using a microscope at five random views.
Assessment of tumorigenicity in vivo
All kinds of treated H1299 and SPC-A1 cells were harvested (1 × 106cells in 150 μl) and injected subcutaneously in athymic nude mice. Mice were divided into three groups, including H1299/SPC-A1, H1299/SPC-A1-BAD overexpression (H1299/SPC-A1-BAD), and BAD-negative control (H1299/SPC-A1-NC). Four weeks later, mice were sacrificed, and tumors were dissected and weighed, then fixed using formalin or stored at −80°C until further use. Tumor volume (mm3) = 0.52 × length (mm) × width (mm) × width (mm). Each group contains 6 mice.
Statistical analysis
Pearson chi-square tests were used to assess the difference in protein expression status among cell lines and tissues. Analysis of variance was performed to determine the statistical significance of differences among the experimental groups. All values were expressed as means ± SD, and Levels of statistical significance were set at P < 0.05 (two-sided). All data were analyzed using SPSS 13.0 for Windows (SPSS Inc., Chicago, Ill, USA).
Results
Overexpression of BAD in NSCLC cell lines inhibits cell proliferation in vitro and tumor growth in vivo
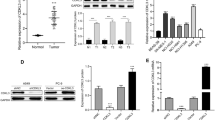
To determine whether high levels of BAD expression contributed to the NSCLC cell proliferation, invasion and apoptosis, we employed a BAD-expressing vector Pdonr223/BAD-pLOV. UBC. EGFP to transfect BAD into NSCLC cell lines, including H1299, H292, A549, H460 and SK-MES. As shown in the Figure 1, expression level of BAD drastically increased in transfected cell groups compared with the non-transfected and empty vector-transfeced cell group (all p < 0.05). In the NC group, BAD expression was not affected by unrelated vector.
Cell proliferation was examined using Cell counting kit-8 at the time points of 1, 2, 3, 4, 5, and 6 days. The cell proliferation rates of the BAD overexpression cell group were significantly decreased compared with NC and normal cell lines in H292, H1299, and SPC-A1, respectively (Figure 2A-C, all p < 0.01), while showed a trend toward lower proliferation rate in H460 cell line, although the difference did not reach statistical significance (Figure 2D, p = 0.077). However, no differences were observed when SK-MES-1 cell line was transfected (Figure 2E, p > 0.05)), suggesting that BAD overexpression inhibited cell proliferation except for squamous cancer cell line. In cell cycle analysis, our results demonstrated that no significant effect on the cell cycle distribution was observed in all cell lines (Figure 3, all p > 0.05).
Effects of BAD overexpression on NSCLC cells cycle. Approximately 5 × 105 to 1 × 106 cells were harvested and stained using propidium iodide. DNA content was determined by flow cytometry. Aggregated cells were gated out and cell cycle profiles were analyzed to quantitate cell cycle distribution. No significant effects on the cell cycle distribution were observed in all cell lines (all p > 0.05).
To better understand the effects of BAD overexpression on NSCLC, xenograft models were established. After subcutaneous injection of H1299/SPC-A1, H1299/SPC-A1-BAD and H1299/SPC-A1-NC cells to the BALB/c-nunu nude mice, tumor volumes were measured every 3 days. Cells in H1299/SPC-A1 and NC group can rapidly form tumors. Nevertheless, BAD overexpression in H1299/SPC-A1 cells reduced tumor formation compared with H1299/SPC-A1 and H1299/SPC-A1-NC control (Figure 4A-B, all p < 0.01). H&E staining showed that xenograft tumor tissues retained major features of the original cancer (Figure 4B). In accordance with the slower growth of H1299/SPC-A1-BAD xenograft, immunohistochemical analysis showed less cells that stained positive for the proliferative marker Ki-67 than those of the controls (Figure 4C-D, all p < 0.01).
BAD overexpression inhibits H1299/SPC-A1 cell tumorigenic activity. Cells (1 × 106 cells in 150 μl) were injected subcutaneously into the backs of BALB/c-nu/nu nude mice. After 4 weeks, tumour weights (A) and volumes (B) were significantly decreased in BAD overexpression groups compared with H1299/SPC-A1-NC and H1299/SPC-A1 groups. The xenograft tumors were processed for H&E staining (C). Representative examples of proliferative marker ki-67 staining in xenograft tumor tissues are shown (D). And data were represented as the mean Ki-67 index ± SD (E). Original magnification, × 200. Arrows indicate positive staining of nuclei. Bar: 50 μm. Data are expressed as the mean ± SD. *: p < 0.05.
Overexpression of BAD has no effect on cell invasion ability
Cell invasion assays were performed to determine the impact of BAD overexpression in NSCLC. We counted invasive cells that transferred to the lower surface of the membrane at five randomly located areas. In all cell types, BAD overexpression had no influence on cell invasion in NSCLC cell types (data not shown, all > 0.05).
Overexpression of BAD induced cell apoptosis in NSCLC cell and xenograft tumors
Apoptotic rates were determined by flow cytometry and TUNEL assays. Our results showed that overexpression of BAD markedly promoted cell apoptosis. In H1299, H292, H460, SPC-A1, and SK-MES-1 cell lines overexpressing BAD, the average apoptotic rates were 19.42%, 23.70%, 41.72%, 3.92%, and 3.12%, respectively, which were higher than those of the control groups (Figure 5A-B, all < 0.05), especially in H460-BAD group (p = 0.000).
Effects of BAD overexpression on NSCLC cell apoptosis. Cells were harvested and stained with V-APC/7AAD. Apoptotic rates were caucluated by flow cytometry (A). The Annexin V-positive and 7AAD-negative cells (Q4) were regarded as apoptotic (B). For the xenograft mice, paraffin-embedded sections of H1299 and SPC-A1 tumor were subjected to TUNEL assay. The amount of TUNEL fluorescence in five random view was quantified (C, all p < 0.05). Representative photomicrographs of TUNEL staining were shown (D). Apoptotic neuclei showed red staining (top), and nuclei stained with DAPI in blue (bottom). The merged images of nuclei in pink (arrows) indicate apoptotic cells. Original magnification, × 200, Bar: 50 μm.
For the xenograft mice, paraffin-embedded sections of H1299 and SPC-A1 tumor were also subjected to TUNEL assay. As shown in Figure 5D, apoptotic cells showed red staining using an inverted fluorescence microscope. The results indicated that the apoptotic cell rates were higher in H1299-BAD and SPC-A1-BAD groups than the controls by >2-fold (Figure 5C, p < 0.05). All data suggested BAD overexpression significantly enhanced tumor cell apoptosis.
Overexpression of BAD enhanced the cyto-c and caspase 3 expressions
In xenograft tumor tissues, the expression levels of BAD were increased in overexpression groups compared with H1299/SPC-A1 and H1299/SPC-A1-NC cell groups (Figure 6A-B).
The expressions of Bcl-2 family proteins and apoptotic related proteins after BAD overexpression. Western blot analysis confirmed that BAD were overexpressed in xenograft model (A-B). The expression levels of Bcl-2, Bcl-xl, Bax, caspase 3, caspase 8 and cyto-c after BAD overexpression were analyzed (C-D, from top to bottom). β-Actin was used to normalize relative expressions among groups.
As BAD is one of core members of the Bcl-2 family, we further analyzed the effect of BAD on expression of other Bcl-2 family members, including Bcl-xl, Bcl-2, Bax, however, all protein above remained unchanged. Meanwhile, expression levels of apoptosis related proteins, such as cyto-c, caspase-3, and caspase-8, were also determined by western blot. Among the proteins aforementioned, cyto-c and caspase 3 expressions were significantly higher in the BAD overexpression group than the controls (all p < 0.05); whereas the other proteins showed similar expression levels in different groups (Figure 6C-D, all p > 0.05).
Discussion
BAD, an important regulator of the cell death machinery, has been reported to contribute to tumorigenesis in several cancers [10, 15, 16]. Loss-of-function models of BAD protein are associated with increased incidence of tumors, and over time, BAD-deficient mice show an increased death rate from tumors [17]. In light of these findings, tumor therapy with BAD protein up-regulation may be warranted. In the present study, we demonstrated that up-regulation of BAD significantly reduced cell proliferation in H1299, H292 and SPC-A1 cell lines in vitro and H1299/SPC-A1 cell tumor growth in vivo, but has no appreciable effects on cell cycle distribution and invasion (data not shown, all p > 0.05) in all cell lines. BAD overexpression also induced apoptosis in all cell types, of which process cyto-c and caspase 3 was involved.
BAD protein, as one of cell death initiators, constitutes a critical control point in apoptosis following cellular damage. Loss of BAD expression alone may promote tumorigenesis due to lack of apoptosis and accumulation of cells with tumorigenic potential. In our study, overexpression of BAD was able to greatly increase the apoptotic rates of NSCLC cell in cultured cells and xenograft tissues, confirming its proapoptotic nature. These are keeping with the report by Mork et al. [18] that BAD act as a key regulator of T cell apoptosis and BAD transgenic mice have depleted numbers of T cells by enhancing sensitive to apoptotic stimuli. Agshin et al. [19] also found that prostatic carcinoma cell line LNCaP, which was resistant to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis, became sensitive to TRAIL and promote apoptotic rate after overexpression of full-length, wild-type BAD. [18]. However, the apoptotic effect of BAD protein overexpression is still controversial. Several studies have shown that overexpression of BAD alone in a cell line has no effect on apoptosis, whereas coexpressing of BAD and Bcl-xl significantly influence cell apoptosis, suggesting that the action of BAD is dependent on heterodimerization with Bcl-xl [20, 21]. Thus, we proposed that the effect of BAD on apoptosis is a result of regulation and interaction with other Bcl-2 family members, including Bcl-xl, Bcl-2, and Bax. Unexpectedly, all these protein expression levels were unchanged. These indicated that BAD could function as a powerful regulator of apoptosis in NSCLC cell independent of Bcl-xl/Bcl-2/Bax interactions or expressions.
Two major apoptotic pathways, the death receptor (extrinsic pathway) and the mitochondrial pathway (intrinsic pathway), have been well characterized in mammalian cells. Over the cause of these pathways, activation of the death receptor first triggers caspase-8 activation, whereas the release of mitochondrial cyto-c activates caspases-9 as an initial caspase 9, all of which subsequently induced the activation of effectors caspases, such as caspase 3 [22, 23]. Cyto-c is an important mitochondrial protein that induces apoptosis when accumulated in the cytosol in response to diverse stress stimuli [24]. In our study, the results revealed that cyto-c and caspase 3 were increased by overexpressing BAD, whereas caspase 8 did not change. These indicated that BAD overexpression-induced apoptosis is associated with cyto-c releasing from mitochondrial, independent of caspase 8 activation. These are in accordance with the studies by Cheng [25] and Wei [26] that BH3 domain-only molecules, including BAD, were required for the disruption of mitochondrial and intrinsic death of cancer cells [27]. Thus, we suggested that BAD overexpression in NSCLC led cancer cells to undergo apoptosis through a mitochondrial pathway.
The pro-apoptotic Bcl-2 family proteins Bax and Bak have been shown to be required for the disruption of mitochondrial and intrinsic death of cancer cells, where as the antiapoptotic Bcl-2 family proteins (Bcl-2 and Bcl-xl) can prevent cell death by interfering with the activation of Bax and Bak [22]. In the present study, we found that the expression levels of Bax, Bcl-2 and Bcl-xl were not changed after BAD overexpression. These suggested that the Bad overexpression-induced mitochondrial pathway was independent of Bax, Bcl-2 and Bcl-xl expression levels.
Recent investigations suggest that functions of the proapoptotic protein BAD are not limited regulating apoptosis [21]. We also found that high level of BAD protein inhibited cell proliferation in H1299, H292, SPC-A1 cell line, and reduced H1299 tumor growth rate in immunocompetent mice. Further immunohistochemical analysis showed that xenograft tumor with BAD overexpression had a decreased number of cells that stained positive for the proliferative marker Ki-67. For H460, a large cell lung cancer cell line, the result also showed a trend toward lower proliferation rate, although the difference did not reach statistical significance. All these indicated that BAD play a negative role in specific cell types, especially in adenocarcinoma cells. This is keeping with our previous report that overexpression of BAD suppressed cell proliferation in another lung adenocarcinoma cell line A549 [12]. In breast cancer cell MCF7, cell growth was also inhibited by BAD overexpression [21]. Contradictive reports appeared that increased BAD expression stimulates proliferation of prostate cancer cells [28]. Knockdown of BAD also led to marked inhibition of proliferation in A375 and SK-MEL-28 malignant melanoma cells, and this growth inhibition could be abrogated by overexpression of wild type BAD [29]. Additionally, in our study, no differences of proliferation were observed in SK-MES-1 squamous cell lung cancer cell. These different results indicating that the effect of BAD on cell proliferation may be cell-type-specific.
To further characterize the mechanism underling growth inhibition, we performed cell cycle analysis. Publications from the Vogt and Yang laboratories have suggested that BAD protein can be involved in promoting cell cycle progression in fibroblast [28, 30, 31]. On the contrary, our results showed that overexpression of BAD did not influence cell cycle distribution in all NSCLC cells. These suggested that, in NSCLC, BAD inhibited cell proliferation in vitro and tumor growth in vivo through direct induction of apoptosis without affecting cell cycle progression.
In cell invasion analysis, our data demonstrated that BAD overexpression had no influence on cell invasion in NSCLC cell types. In contrast, a previous AACR symposium poster [32] reported that BAD inhibited cancer cell invasion in breast cancer. From now on, there are very limited reports of the effects of BAD on cell invasion. These inconsistencies remained to be confirmed in expanded and intensive studies.
Conclusions
In conclusion, this study extended our previous findings that BAD expression level was an independent poor prognostic marker in NSCLC patients. BAD overexpression alone induces cell apoptosis, and depressed cell proliferation and cell growth depends on cell types, especially in adenocarcinoma. In the further investigation, BAD may function as tumor suppressor regulating cell growth and apoptosis in the development of NSCLC, and is a potential target for tumor intervention.
Abbreviations
- NSCLC:
-
Non-small cell lung cancer
- cyto-c:
-
Cytochrom c.
References
Selinger CI, Cooper WA, Al-Sohaily S, Mladenova DN, Pangon L, Kennedy CW, McCaughan BC, Stirzaker C, Kohonen-Corish MR: Loss of special AT-rich binding protein 1 expression is a marker of poor survival in lung cancer. J Thorac Oncol. 2011, 6: 1179-1189. 10.1097/JTO.0b013e31821b4ce0.
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 2009, 59: 225-249. 10.3322/caac.20006.
Huang Y, Liu D, Chen B, Zeng J, Wang L, Zhang S, Mo X, Li W: Loss of Bad expression confers poor prognosis in non-small cell lung cancer. Med Oncol. 2012, 29: 1648-1655. 10.1007/s12032-011-0060-4.
Downward J: How BAD phosphorylation is good for survival. Nat Cell Biol. 1999, 1: E33-E35. 10.1038/10026.
Kuroda J, Taniwaki M: Involvement of BH3-only proteins in hematologic malignancies. Crit Rev Oncol Hematol. 2009, 71: 89-101. 10.1016/j.critrevonc.2008.10.004.
Adams JM, Cory S: The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007, 26: 1324-1337. 10.1038/sj.onc.1210220.
Sommer P, Cowen RL, Berry A, Cookson A, Telfer BA, Williams KJ, Stratford IJ, Kay P, White A, Ray DW: Glucocorticoid receptor over-expression promotes human small cell lung cancer apoptosis in vivo and thereby slows tumor growth. Endocr Relat Cancer. 2010, 17: 203-213. 10.1677/ERC-09-0241.
Ranger AM, Zha J, Harada H, Datta SR, Danial NN, Gilmore AP, Kutok JL, Le Beau MM, Greenberg ME, Korsmeyer SJ: Bad-deficient mice develop diffuse large B cell lymphoma. Proc Natl Acad Sci U S A. 2003, 100: 9324-9329. 10.1073/pnas.1533446100.
Gilmore AP, Valentijn AJ, Wang P, Ranger AM, Bundred N, O’Hare MJ, Wakeling A, Korsmeyer SJ, Streuli CH: Activation of BAD by therapeutic inhibition of epidermal growth factor receptor and transactivation by insulin-like growth factor receptor. J Biol Chem. 2002, 277: 27643-27650. 10.1074/jbc.M108863200.
Sinicrope FA, Rego RL, Foster NR, Thibodeau SN, Alberts SR, Windschitl HE, Sargent DJ: Proapoptotic Bad and Bid protein expression predict survival in stages II and III colon cancers. Clin Cancer Res. 2008, 14: 4128-4133. 10.1158/1078-0432.CCR-07-5160.
Seow HF, Yip WK, Loh HW, Ithnin H, Por P, Rohaizak M: Immunohistochemical detection of phospho-Akt, phospho-BAD, HER2 and oestrogen receptors alpha and beta in Malaysian breast cancer patients. Pathol Oncol Res. 2011, 16: 239-248.
Huang N, Zhu J, Liu D, Li YL, Chen BJ, He YQ, Liu K, Mo XM, Li WM: Overexpression of Bcl-2-associated death inhibits A549 cell growth in vitro and in vivo. Cancer Biother Radiopharm. 2012, 27: 164-168. 10.1089/cbr.2011.1018.
Liu D, Huang Y, Zeng J, Chen B, Huang N, Guo N, Liu L, Xu H, Mo X, Li W: Down-regulation of JAK1 by RNA interference inhibits growth of the lung cancer cell line A549 and interferes with the PI3K/mTOR pathway. J Cancer Res Clin Oncol. 2011, 137: 1629-1640. 10.1007/s00432-011-1037-6.
Liu D, Huang Y, Chen B, Zeng J, Guo N, Zhang S, Liu L, Xu H, Mo X, Li W: Activation of mammalian target of rapamycin pathway confers adverse outcome in nonsmall cell lung carcinoma. Cancer. 2011, 117: 3763-3773. 10.1002/cncr.25959.
Al-Bazz YO, Underwood JC, Brown BL, Dobson PR: Prognostic significance of Akt, phospho-Akt and BAD expression in primary breast cancer. Eur J Cancer. 2009, 45: 694-704. 10.1016/j.ejca.2008.11.044.
Marchion DC, Cottrill HM, Xiong Y, Chen N, Bicaku E, Fulp WJ, Bansal N, Chon HS, Stickles XB, Kamath SG, Hakam A, Li L, Su D, Moreno C, Judson PL, Berchuck A, Wenham RM, Apte SM, Gonzalez-Bosquet J, Bloom GC, Eschrich SA, Sebti S, Chen DT, Lancaster JM: BAD phosphorylation determines ovarian cancer chemosensitivity and patient survival. Clin Cancer Res. 2012, 17: 6356-6366.
Danial NN: BAD: undertaker by night, candyman by day. Oncogene. 2008, 27 (Suppl 1): S53-S70.
Mok CL, Gil-Gómez G, Williams O, Coles M, Taga S, Tolaini M, Norton T, Kioussis D, Brady HJ: Bad can act as a key regulator of T cell apoptosis and T cell development. J Exp Med. 1999, 189: 575-586. 10.1084/jem.189.3.575.
Taghiyev AF, Guseva NV, Harada H, Knudson CM, Rokhlin OW, Cohen MB: Overexpression of BAD potentiates sensitivity to tumor necrosis factor-related apoptosis-inducing ligand treatment in the prostatic carcinoma cell line LNCaP. Mol Cancer Res. 2003, 1: 500-507.
Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ: Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995, 80: 285-291. 10.1016/0092-8674(95)90411-5.
Fernando R, Foster JS, Bible A, Strom A, Pestell RG, Rao M, Saxton A, Baek SJ, Yamaguchi K, Donnell R: Breast cancer cell proliferation is inhibited by BAD: regulation of cyclin D1. J Biol Chem. 2007, 282: 28864-28873. 10.1074/jbc.M700785200.
Lee KW, Kim SG, Kim HP, Kwon E, You J, Choi HJ, Park JH, Kang BC, Im SA, Kim TY, Kim WH, Bang YJ: Enzastaurin, a protein kinase C beta inhibitor, suppresses signaling through the ribosomal S6 kinase and bad pathways and induces apoptosis in human gastric cancer cells. Cancer Res. 2008, 68: 1916-1926. 10.1158/0008-5472.CAN-07-3195.
Hengartner MO: The biochemistry of apoptosis. Nature. 2000, 407: 770-776. 10.1038/35037710.
Chauhan D, Pandey P, Ogata A, Teoh G, Krett N, Halgren R, Rosen S, Kufe D, Kharbanda S, Anderson K: Cytochrome c-dependent and -independent induction of apoptosis in multiple myeloma cells. J Biol Chem. 1997, 272: 29995-29997. 10.1074/jbc.272.48.29995.
Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer SJ: BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001, 8: 705-711. 10.1016/S1097-2765(01)00320-3.
Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ: Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001, 292: 727-730. 10.1126/science.1059108.
Xiao D, Singh SV: Diallyl trisulfide, a constituent of processed garlic, inactivates Akt to trigger mitochondrial translocation of BAD and caspase-mediated apoptosis in human prostate cancer cells. Carcinogenesis. 2006, 27: 533-540.
Smith AJ, Karpova Y, D’Agostino R, Willingham M, Kulik G: Expression of the Bcl-2 protein BAD promotes prostate cancer growth. PLoS One. 2009, 4: e6224-10.1371/journal.pone.0006224.
Polzien L, Baljuls A, Albrecht M, Hekman M, Rapp UR: BAD contributes to RAF-mediated proliferation and cooperates with B-RAF-V600E in cancer signaling. J Biol Chem. 2011, 286: 17934-17944. 10.1074/jbc.M110.177345.
Maslyar DJ, Aoki M, Vogt PK: The growth-promoting activity of the Bad protein in chicken embryo fibroblasts requires binding to protein 14-3-3. Oncogene. 2001, 20: 5087-5092. 10.1038/sj.onc.1204662.
Chattopadhyay A, Chiang CW, Yang E: BAD/BCL-[X(L)] heterodimerization leads to bypass of G0/G1 arrest. Oncogene. 2001, 20: 4507-4518. 10.1038/sj.onc.1204584.
Cekanova M, Fernando R, Wimalasena J: P1-02-11: the BCL2 antagonist of death, BAD is down-regulated in breast cancer and inhibits cancer cell invasion. 2011, Abstracts: Thirty-Fourth Annual CTRC-AACR San Antonio Breast Cancer Symposium
Acknowledgments
This work was supported by grants from the Nature Science Foundation of China (grant 81241068 to WM.L. and grant 81201851 to D.L.) and Sichuan Science and Technology Department (grant 2001SZ0 194 and 2011HH0051 to WM.L.).
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JL and Man LM carried out most of the experimental studies. LD participated in designing the research, performed statistical analyses and drafted the manuscript. CBJ, ZW and ML participated in the animal and cell experiments. ZJ and HN participated in western blot assays. HY performed statistical analyses and drafted the manuscript. MXM revised the manuscript. LWM designed the research, supervised the experiments and completed the manuscript. All authors read and approved the final manuscript.
Li Jiang, Man Luo, Dan Liu contributed equally to this work.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Jiang, L., Luo, M., Liu, D. et al. BAD overexpression inhibits cell growth and induces apoptosis via mitochondrial-dependent pathway in non-small cell lung cancer. Cancer Cell Int 13, 53 (2013). https://doi.org/10.1186/1475-2867-13-53
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1475-2867-13-53