Abstract
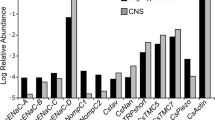
It is known that endogenous mechanical stress in the tissues of developing organisms is involved in regulating both morphogenesis and cell differentiation. These processes can be regulated by bioelectrical signals. Mechanosensitive ion channels are the structures that transform the mechanical stimulus into the bioelectrical signal. In this work we study the expression of mechanosensitive TRP ion channels during early development of Xenopus tropicalis. Real-time RT-PCR and PCR show the spatio-temporal expression of mRNA of mechanosensitive TRP ion channels during the early development of Xenopus tropicalis.
Similar content being viewed by others
References
Farge E. 2003. Mechanical induction of twist in the Drosophila foregut/stomodeal primordium. Curr. Biol. 13, 1365–1377.
Beloussov L.V., Luchinskaia N.N., Ermakov A.S., Glagoleva N.S. 2006. Gastrulation in amphibian embryos, regarded as a succession of biomechanical feedback events. Int. J. Dev. Biol. 50, 113–122.
Kornikova E.S., Troshina T.G., Kremnyov S.V., Beloussov L.V. 2010. Neuro-mesodermal patterns in artificially deformed embryonic explants: A role for mechano-geometry in tissue differentiation. Dev. Dyn. 239 (3), 885–896.
McBeath R., Pirone D.M., Nelson C.M., Bhadriraju K., Chen C.S. 2004. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell. 6, 483–495.
Engler A.J., Sen S., Sweeney H.L., Discher D.E. 2006. Matrix elasticity directs stem cell lineage specification. Cell. 126, 677–689.
Beloussov L.V., Kazakova N.I., Luchinskaia N.N., Novoselov V.V. 1997. Studies in developmental cytomechanic. Int. J. Dev. Biol. 41, 793–799.
Beloussov L.V., Dorfman J.G., Cherdantzev V.G. 1975. Mechanical stresses and morphological patterns in amphibian embryos. J. Embryol. Exp. Morphol. 3, 559–574.
Beloussov L.V., Lakirev A.V., Naumidi I.I., Novoselov V.V. 1990. Effects of relaxation of mechanical tensions upon the early morphogenesis of Xenopus laevis embryos. Int. J. Dev. Biol. 34, 409–419.
Pai V.P., Aw S., Shomrat T., Lemire J.M., Levin M. 2012. Transmembrane voltage potential controls embryonic eye patterning in Xenopus laevis. Development. 139 (2), 313–323.
Levin M., Stevenson C.G. 2012. Regulation of cell behavior and tissue patterning by bioelectrical signals: Challenges and opportunities for biomedical engineering. Annu. Rev. Biomed. Eng. 14, 295–323.
Plant T.D. 2014. TRPs in mechanisensing and volume regulation. Handbook Exp. Pharmacol. 223, 743–766.
Inoue R., Jian Z., Kawarabayashi Y. 2009. Mechanosensitive TRP channels in cardiovascular pathophysiology. Pharmacol. Therapeutics. 123 (3), 371–385.
Thodeti C.K., Matthews B., Ravi A., Mammoto A., Ghosh K., Bracha A.L., Ingber D.E. 2009. TRPV4 channels mediate cyclic strain-induced endothelial cell reorientation through integrin-to-integrin signaling. Circ Res. 104 (9), 1123–1130.
Takao D., Nemoto T., Abe T., Kiyonari H., KajiuraKobayashi H., Shiratori H., Nonaka S. 2013. Asymmetric distribution of dynamic calcium signals in the node of mouse embryo during left–right axis formation. Dev Biol. 376 (1), 23–30.
Fabian A., Bertrand J., Lindemann O., Pap T., Schwab A. 2012. Transient receptor potential canonical channel 1 impacts on mechanosignaling during cell migration. Pflügers Arch. 464, 623–630.
Chalfie M. 2009. Neurosensory mechanotransduction. Nat. Rev. Mol. Cell. Biol. 10, 44–52.
Gillespie P.G., Walker R.G. 2001. Molecular basis of mechanosensory transduction. Nature. 413, 194–202.
Hamill O.P. 2006. Twenty odd years of stretch-sensitive channels. Pflügers Arch. 453, 333–351.
Kung C. 2005. A possible unifying principle for mechanosensation. Nature. 436, 647–654.
Kung C., Martinac B., Sukharev S. 2010. Mechanosensitive channels in microbes. Annu. Rev. Microbiol. 64, 313–329.
Nilius B., Honore E. 2012. Sensing pressure with ion channels. Trends Neurosci. 35, 477–486.
Sharif-Naeini R., Dedman A., Folgering J.H., Duprat F., Patel A., Nilius B., Honore E. 2008. TRP channels and mechanosensory transduction: insights into the arterial myogenic response. Pflügers Arch. 456, 529–540.
Sukharev S., Sachs F. 2012. Molecular force transduction by ion channels: Diversity and unifying principles. J. Cell. Sci. 125, 3075–3083.
Ogino H., McConnell W.B., Grainger R.M. 2006. Highly efficient transgenesis in Xenopus tropicalis using I-SceI meganuclease. Mech. Dev. 123 (2), 103–113.
Bookout A., Cummins C., Mangelsdorf D. 2005. Current protocols in molecular biology. Hoboken: John Wiley and Sons, Inc. P. 15.8.1–15.8.21.
Ramakers C., Ruijter J.M., Deprez R.H.L., Moorman A.F.M. 2003. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 339 (1), 62–66.
Nieuwkoop P.D., Faber J. 1956. Normal table of Xenopus laevis (Daudin). Amsterdam: North-Holland Publications.
Newby L.J., Streets A.J., Zhao Y., Harris P.C., Ward C.J., Ong A.C. 2002. Identification, characterization, and localization of a novel kidney polycystin-1-polycystin-2 complex. J. Biol. Chem. 277, 20763–20773.
Heasman J. 2006. Patterning the early Xenopus embryo. Development. 133 (7), 1205–1217.
Maroto R., Raso A., Wood T.G., Kurosky A., Martinac B., Hamill O.P. 2005. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat. Cell. Biol. 7 (2), 179–185.
Köttgen M., Buchholz B., Garcia-Gonzalez M.A., Kotsis F., Fu X., Doerken M., Boehlke C., Steffl D., Tauber R., Wegierski T., Nitschke R., Suzuki M., Kramer-Zucker A., Germino G.G., Watnick T., Prenen J., Nilius B., Kuehn E.W., Walz G. 2008. TRPP2 and TRPV4 form a polymodal sensory channel complex. J. Cell Biol. 182 (3), 437–447.
Tsiokas L., Arnould T., Zhu C., Kim E., Walz G., Sukhatme V.P. 1999. Specific association of the gene product of PKD2 with the TRPC1 channel. Proc. Natl. Acad. Sci. USA. 96 (7), 3934–3939.
Du J., Ma X., Shen B., Huang Y., Birnbaumer L., Yao X. 2014. TRPV4, TRPC1 and TRPP2 assemble to form a flow-sensitive heteromeric channel. FASEB J. 28 (11), 4677–4685.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © S.G. Silina, D.A. Nikishin, S.V. Kremnyov, 2015, published in Biologicheskie Membrany, 2015, Vol. 32, No. 3, pp. 194–201.
Rights and permissions
About this article
Cite this article
Silina, S.G., Nikishin, D.A. & Kremnyov, S.V. Spatio-temporal expression pattern of mechanosensitive TRP ion channels during early development of Xenopus tropicalis . Biochem. Moscow Suppl. Ser. A 9, 194–201 (2015). https://doi.org/10.1134/S1990747815020191
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747815020191