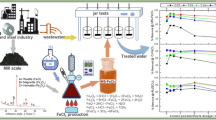
Abstract
New single-stage method was developed for chemical utilization of wastewater containing 0.25 to 400 g L–1 of chromium in terms of chromium anhydride with the us of sulfuric acid and steel cuttings. The method makes it possible to convert hexavalent chromium into easily used ferrochromium precipitates. It was found that there occur periodic synchronous concentration fluctuations of Cr(VI) and Cr(III) up to, respectively, 400 g L–1 and 150 mg L–1 in the course of reduction of hexavalent chromium with the use of steel cuttings in sulfuric acid solutions.
Similar content being viewed by others
References
Vinogradov, S.S., Ekologicheski bezopasnoe gal’vanicheskoe proizvodstvo (Ecologically Safe Electroplating Industry), Kudryavtsev, V.N., Ed., Moscow: Globus, 2002, 2nd ed. rev. suppl.
Kuzubova, L.I. and Morozov, S.V., Ochistka neftesoderzhashchikh stochnykh vod: Analiticheskii obzor (Purification of Oil-containing Wastewater: Analytical Review), Novosibirsk: SORAN; GPNTB; NIOKh, 1992.
Kvasnikov, E.I. and Serpokrylov, N.S., Biologicheskaya ochistka khromsoderzhashchikh promyshlennykh stochnykh vod (Biological Purification of Chromium-containing Industrial Wastewater), Kiev: Naukova Dumka, 1990.
Chanturiya, V.A. and Solozhenkin, P.M., Gal’vanokhimicheskie metody ochistki tekhnogennykh vod (Galvanochemical Methods for Purification of Technological Wastewater), Moscow: IKTs Akademkniga, 2005.
Vinogradov, S.S. and Kruglikov, S.S., Gal’vanotekh. Obrab. Poverkh., 2008, vol. 16, no. 1, pp. 46–47.
Skrylev, L.D., Skryleva, T.L., and Koltykova, G.N., Khim. Tekhnol. Vody, 1997, vol. 19, no. 5, pp. 516–523.
Rogov, V.M. and Shvetsova, T.L., Khim. Tekhnol. Vody, 1986, vol. 8, no. 3, pp. 22–25.
Fazlutdinov, K.K., Markov, V.F., and Maskaeva, L.N., Butlerov Soobshch., 2014, vol. 37, no. 2, pp. 10–17.
Fazlutdinov, K.K., Markov, V.F., and Maskaeva, L.N., Butlerov Soobshch., 2014, vol. 39, no. 8, pp. 34–39.
Meites, L., An Introduction to Chemical Equilibrium and Kinetics, New York: Pergamon Press, 1981.
Nikandrova, L.I., Gerasimova, N.I., Ivanova, L.V., and Kondratovich, G.A., Analiz elektrolitov i rastvorov (Analysis of Electrolytes and Solutions), Moscow: Gos. Khim. Izdat, 1963.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © K.K. Fazlutdinov, V.F. Markov, A.V. Gorokhov, L.N. Maskaeva, 2016, published in Zhurnal Prikladnoi Khimii, 2016, Vol. 89, No. 8, pp. 971−977.
Rights and permissions
About this article
Cite this article
Fazlutdinov, K.K., Markov, V.F., Gorokhov, A.V. et al. Utilization of Cr(VI) solutions with steel shavings. Russ J Appl Chem 89, 1221–1227 (2016). https://doi.org/10.1134/S1070427216080024
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070427216080024