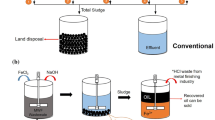
Abstract
Mill scale (MS) is considered to be a significant metallurgical waste, but there is no economical method yet to utilize its metal content. In this study, which covers various processes in several stages, the solution of iron in MS, which is the Iron and Steel Industry (I&SI) waste, as FeCl3 (MS-FeCl3) in the thermoreactor in the presence of HCl, was investigated. In the next step, the conditions for using this solution as a coagulant in the treatment of I&SI wastewater were investigated using the jar test. The results of the treated water sample were compared by chemical oxygen demand (COD), total suspended solids (TSS), color, and turbidity analyses using commercial aluminum sulfate (Al2(SO4)3) and FeCl3 (C-FeCl3). Additionally, heavy metal analyses were conducted, and the treatment performance of three coagulants was presented. Accordingly, while 2.0 mg/L anionic polyelectrolyte was consumed at a dosage of 4.05 mg/L Al2(SO4)3 at pH 7.0, 0.25 mg/L anionic polyelectrolyte was consumed at a dosage of 1.29 mg/L at pH 5.0 in the C-FeCl3 and MS-FeCl3 studies. Also, Fe, Cr, Mn, Ni, Zn, Cd, Hg, and Pb removal efficiencies were over 93.56% for all three coagulant usage cases. The results showed that the wastewater treatment performance of MS-FeCl3 by the recycling of MS, which is an I&SI waste, was at the same level as C-FeCl3. Thus, thanks to recycling, waste scale can be used as an alternative to commercial products for green production.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Cooling the billet/plate released to the market in at rolling mill unit within the I&SI is a common method. Meanwhile, oxide layers appear in the high-temperature and oxygen range. This layer is called scale and is classified as waste (Khaerudini et al. 2019). This oxide layer is removed using high-pressure water from the MS formed on the surface due to the high-temperature (1100–1300°C) and oxidizing environment during the production phase (Önkibar 2006; Gündoğdu 2013). Most of the MS is composed of iron oxide structures such as wustite (FeO), magnetite (Fe3O4), and hematite (Fe2O3) (Turan et al. 2021). The scale typically contains around 70% Fe (El-Hussiny et al. 2014). Scale is known as an important metallurgical waste, but there is no technology yet to use it economically. On the other hand, in recent years, mostly since the tremendous growth of the I&SI, MS has become a valuable material. MS is now collected and sold for reuse in many industrial applications (Sanin et al. 2019). Although the exact rate is unknown in rolling mills worldwide, 2% of steel production is assumed to involve be scale. If the world’s steel production is 1.950 billion tons, approximately 39 million tons of scale is formed per year (Jikar and Dhokey 2021; World Steel Association 2022). Scale is briquette in integrated iron and steel plants, and it is used with iron ore in basic oxygen furnaces (BOF) and pelletizing plants (Lisin et al. 2003; Baryshev et al. 2011; Osman 2012). By reducing the iron in scale, iron powder (Sen et al. 2015) and Fe3O4 are produced (Shahid et al. 2019). Additionally, it is possible to encounter the use of scale as a reinforcement material in low-cost ceramic production (Bantsis et al. 2012), electromagnetic wave protectors (Ozturk et al. 2020), and concrete and brick production (Baghel et al. 2020). A part of MS is directly recycled within the steelmaking industry and amounts are used in ferroalloys, cement plants, road construction, to make tiles, to prepare some iron oxide pigments, and in the petrochemicals industry (Iluiu-Varvara et al. 2020). MS contains high amounts of iron. Therefore, it can be used to recover iron by mixing it with industrial waste and other by-products to create the raw material used during the production process. In these applications, MS is processed by roasting, leaching and other processes. As a result, the utilization of the scale formed in I&SI is a common problem of similar industries worldwide. Since the recycling of scale allows the reduction of raw material dependency and the improvement of the ecology, reusing scale is becoming more important due to the constant increase in the cost of iron ore. Therefore, there is a great trend towards recovering valuable iron content in the production process. It has become a subject in recent years that researchers frequently focus on.
I&SI are industrial establishments that use many raw material resources and release high amounts of solid, liquid, and gaseous wastes to the environment in the same proportion. The aforementioned industrial sector wastewaters contain significant amounts of heavy metals originating from various production or cooling processes, and the most common metals: Fe, Pb, Cd, Cu, and Zn (Ahmaruzzaman 2011; Iftikhar et al. 2023; Pang et al. 2023). In the process of pig iron production at high temperatures, there is a large amount of water consumption in the cooling section, steel shaping sections, and coating units due to the nature of the process. Before the actual processing step of steel production, clean water is supplied to the water supply system and then transported to steelmaking plant sections such as coking, sintering, blast furnace, steel mill, hot rolling mill, and cold rolling mills sections. While a small part of the water is consumed in the process in the factory, a large part of it is sent at the wastewater treatment plant, where it is treated and classified according to the treatment method used. If the discharged wastewater can be improved and reused, serious benefits can be achieved in terms of sustainability.
The steel production in the world in 2022 amount to 1.95 billion tons, and Turkey ranks 7th in the world and 1st in Europe with 40.4 million tons of steel produced (SteelData 2023). It is assumed that water reuse, water consumption, and water discharge per ton of steel in iron and steel plants are 14.93 t, 5.90 t, and 9.23 t, respectively (Tong et al. 2019). While the world’s water consumption per ton of steel is between 3 and 6 m3, it can reach 25–60 m3 in less developed countries (Sirajuddin et al. 2010). Process water per ton of raw steel in Turkey’s I&SI varies between 3.5 and 15 m3. In this regard, in the sections of plants where water is consumed in the process, after a few cycles (varying depending on the production amount and raw material input), the water is sent to a wastewater treatment plant.
I&SI cooling water and water originating from other washing processes contain metal particles, suspended solids, oil-grease, organic matter, ammonia, surfactant, cyanide, fluoride, metal, heavy metal, and especially ferrous ions (Biswas 2013; Mahjouri et al. 2017; Phan Quang et al. 2022). The pollutants in these wastewaters are treated by adsorption (Beh et al. 2012), the Fenton process (Phan Quang et al. 2022), the membrane filtration method (Changmai et al. 2020; Zhang et al. 2022b), ultrafiltration and reverse osmosis (Sun et al. 2019), and coagulation-flocculation processes (Garg and Singh 2022). The net surface charges are reduced by adding metal salts to the negatively charged particles repel each other in water that. With sufficient Van der Walls forces, the pollutants are precipitated by reducing the surface area and increasing their weight by allowing them to hold onto each other. This process is known as the coagulation process. It is known that among the treatment processes listed above, scale-up is preferred more than other processes in terms of cost and the ease of application (Abujazar et al. 2022a). Coagulation is performed by adding chemicals such as Al2(SO4)3, FeCl3, ferric sulfate, poly aluminum chloride (PACl), and polyaluminum ferric chloride (PAFCl) as positively charged metal salts (Verma et al. 2012). Polyelectrolytes ensure that are formed are tightened, their molecular weights increase, and flocs are formed. These flocs are removed from the medium by filtration, flotation, or sedimentation (Bratby 2016; Abujazar et al. 2022a). Moreover, in the metal industry, this process is used for the removal of organic and inorganic pollutants from oil and petrochemical industry wastewater (Zhao et al. 2021), the removal of heavy metals such as Cu2+, Cr6+, Cd2+, and Pb2+ from many different wastewaters (Fu and Wang 2011), color removal from textile wastewater (Verma et al. 2012), and paper recycling wastewater (Mainardis et al. 2022) and the removal of microplastics (Tang et al. 2022).
While wastewater is created in high amounts in terms of the flow rate in I&SI, it has a negative impact on the receiving environment due to the multiple pollutants (organic wastes and heavy metals) it contains (Mondal et al. 2019; Sun et al. 2019; Garg and Singh 2022). The coagulation/flocculation process, which is one of the safest and most frequently used methods for the treatment of these wastewaters, is a preferred operation. This process requires salts/solutions of metals such as Fe and Al (Das et al. 2018). Like almost all industries, the main goal of I&SI is to be sustainable, especially by reducing the amount of solid waste it produces (Mondal et al. 2019). In this context, the conversion of scale, which contains high amounts of Fe, into FeCl3 by thermochemistry and its removal from the wastewater into which it is discharged will make a significant contribution to the basic sustainability targets of this industry.
In this respect, it is clear that various salt compounds based on iron and aluminum are used in the treatment of industrial wastewater today. Examples of these include the iron and steel industry (Jung et al. 2006; Colla et al. 2016; Vasilenko and Koltun 2017), the galvanic, tinned and colored steel sheet processing process (Taheriyoun et al. 2020), and the coating sector from metal side branches. It was treated using metal salts and derivatives (Al and Fe) (Al-Shannag et al. 2015; Oden and Sari-Erkan 2018) in the treatment of wastewater. However, the consumption costs of these products used commercially in treating constitute the most important limitation of the current situation in this field. For this reason, in the study, it was revealed that it is necessary to demonstrate the treated of I&SI wastewater in the presence of Fe3+ ions obtained by the extraction method from MS, without the need for any industrial metal salt compounds, and to compare the treatment efficiency performances and polyelectrolyte consumptions of their commercially available counterparts. In addition, by pioneering the development of a method that can replace the metal salt reactants that are frequently purchased and used commercially with the traditional refining method, environmentally risky smelter wastes have also been evaluated. Thus, the environmental and economic disadvantages caused by solid wastes have been turned into advantages in the treatment of liquid wastes. In addition, due to the limited reuse of MS, the fact that it will be used by producing it as metallic salt solutions will have benefits that will contribute to the reduction of stocks of this substance. Considering that the MS-FeCl3 produced in the process can be used in other research subjects or in the treatment systems of facilities, it will provide a separate added value.
The main purpose of this study is to investigate the usability of MS-FeCl3 coagulant obtained by extraction method from MS in the treatment of I&SI wastewater. For this purpose, the scale formed in the process was converted into FeCl3 and used as a coagulant in the treatment of the wastewater of this industry. The optimum production conditions were determined in the treatment process with HCl, and the treatment performance of the obtained MS-FeCl3 was compared to the performance of Al2(SO4)3 and C-FeCl3, which are widely used. Thus, it has been revealed that MS can be evaluated and used as an alternative coagulant in the treatment of the wastewater in the market.
Material and method
Analytical methods
Chemicals
Aluminum sulfate (Al2(SO4)3.18H2O, 98%, Sigma-Aldrich), iron (III) chloride (FeCl3.6H2O ≥ 98.0%, Merck), anionic polyelectrolyte (poly (2-acrylamido-2-methyl-1-propanesulfonic acid-co-acrylonitrile), ~ 95 wt.%, Sigma-Aldrich), sodium hydroxide (NaOH, ≥ 99.0%, Sigma-Aldrich), sulfuric acid (H2SO4, ≥ 99.99%, Merck), 5-sulfosalicylic acid dihydrate (C7H6O6S.2H2O, ≥ 99.0%, Merck), ethylene diamine tetra acetic acid (EDTA) (C10H14N2Na2O8.2H2O, 95.0%, Sigma-Aldrich), potassium permanganate (KMnO4, 99%, Merck), and hydrochloric acid (HCl, 36%, Sigma-Aldrich) were used in the study. The specifications of the chemicals that are used in the experiments are presented in Table 1.
Analysis of Fe3+ and Fe2+ in the coagulant produced from mill scale
The determination of Fe3+ in the solution obtained at the end of the first-stage experiments was achieved with titrimetric method using the sulfosalicylic acid indicator. In the titration process, EDTA at 99% purity was used. Finally, amount of the spent EDTA solution was noted, and the conversion ratio of dissolved iron to Fe3+ was calculated using Eq. (1) below (Gülensoy 1968).
where CR is the “Conversion Ratio” of Fe3+/dissolved Fe, A is the volume of EDTA spent (mL), B is the initial solution volume (mL), C is the volume of indicator used (mL), D is the volume of solution used (mL), and E is the amount of total iron (mg).
The potassium permanganate titration method determines the ferrous iron concentration in the final product. The method is based on the following reaction:
The ferrous iron concentration can be expressed as below:
where V (mL) is the volume of potassium permanganate consumed at the endpoint, V0 (mL) is the volume of potassium permanganate consumed by distilled water at the endpoint, C is the concentration (M) of the standard potassium permanganate solution, m is the mass (g) of a sample, and 0.5585 is the mass of 0.001 mol iron (Li et al. 2009).
Wastewater sample collection and analysis
Raw wastewater samples were taken from the input point of the central wastewater treatment plant of İskenderun Organized Industrial Zone (Hatay/Turkey), where I&SI wastewater, with a flow rate of approximately 7000 m3/day, which has a physical and chemical treatment process, is collected. The samples were collected and stored in 20-L plastic PE containers at + 4 ℃. pH measurements were made with the WTW 315i pH meter. The color analysis was carried out using a UV–Vis spectrophotometer (Peak E-1000UV, China) in the experiments at 455 nm with to SM 2120 C according to the equation y = 0.00073x-0.007 (R2 0.9996) (AWWA 2017), the TSS analysis was performed according to the equation y = 0.000591x-0.00725 (R2 0.9976) at 810 nm (Karam et al. 2020), and the turbidity analysis was performed at 541 nm using the equation y = 0.000637x-0.01092 (R2 0.9979) (Dotto et al. 2019). Furthermore, the COD analyses were performed at 600 nm according to the equation y = 0.000431x-0.03872 (R2 0.9997) SM5220-D Closed Reflux, Colorimetric Method (AWWA 2017). ICP-MS (Inductively Coupled Plasma Mass Spectrometry-Agilent, 7500A Model) was used to analyze raw and treated wastewater for the presence of heavy metals (Beauchemin 2008).
Experimental procedure
The experiments that were conducted in the study could be divided into three groups. In the first stage, the MS was processed, while the MS was leached HCl, and a solution with FeCl3 was obtained in the second stage investigated. In the third stage, the usage conditions of the coagulant for the obtained solution were examined in the treatment of the I&SI wastewater. Finally, C-FeCl3 and Al2(SO4)3 were obtained to compare their performance to that of the coagulant we produced for use in the third-stage experiments.
First stage: pre-processing of MS
Approximately 15 kg of MS was obtained from the İskenderun Iron and Steel Factory. Due to the presence of oil, the MS was washed in the laboratory with water a few times and kept at room temperature overnight. Next, after drying for 12 h in a stove adjusted to 323 K and then grinding in a ring mill for 2 min, the sample was passed through a − 90-μm sieve and stored in closed containers to be used later in leaching experiments. The chemical concentrations of the MS were determined by solubilization followed by the analysis of the solutions by ICP-OES (Inductively Coupled Plasma Optical Emission Spectrometry- Perkin-Elmer, Optima 2000DV) (Table 2). It was determined that the chemical composition of the MS was mainly included iron (approximately 64% Fe). The mineralogical analyses of the MS mainly carried out by XRD (X-Ray Diffraction- Bruker/D8 Advance), while their morphological structure was characterized using a scanning electron microscope (SEM—Zeiss/EVO MA10). The XRD and SEM–EDX analysis processes of the MS are seen in Fig. 1. According to the results of the XRD analyses of the MS, the structure mainly consisted of FeO, Fe2O3, and Fe3O4. According to the differences obtained in the structural analyses, Fe, which would be the source of the ferric chloride to be obtained from the MS in the presence of HCl, had essentially different oxidation degrees in the MS.
Second stage: production FeCl3 from MS
The first-stage experiments were conducted in a thermoreactor (Hach LT200, Germany) from a heated cylindrical housing. The thermoreactor could be used with 24 borosilicate test tubes with screw cap tubes of at the dimensions 16 × 100 mm. The solutions were prepared by filling a reasonable amount of acid taken from an HCl (37% HCl) stock solution up to 10 mL. At this stage, the effects of various parameters were examined in different ranges for HCl concentration (1–9 M), temperature (318–378 K), and time (15–180 min). After the end of the leaching time, the solution mixture was passed through a blue filter paper. In this study, it was essential to determine the concentration of iron taken into the solution as a result of treating the MS with HCl and on which oxidation step it was. For this reason, in the obtained solutions, Fe3+, Fe2+, and total Fe analyses were carried out, respectively, by the titrimetric method. Serial experiments were carried out in the determined optimum conditions, and the obtained solutions were stored for use in the third stage of the study.
Third stage: batch wastewater treatment procedure
Batch jar test experiments were performed at room temperature using a mixer (Velp, Italy) with six beakers containing 50 mL of I&SI wastewater. The experimental procedure included rapid mixing at 240 rpm for 3 min, slow mixing at 60 rpm for 6 min, and settling for 15 min. Al2(SO4)3, C-FeCl3, and MS-FeCl3 were used as conventional coagulants in the experiments. In the first stage of the coagulation/flocculation study, a 1.0-mg/L flocculant dosage was kept constant, and studies were carried out to determine the optimum pH and optimum coagulant dose for all three coagulants. A total of 0.1 M H2SO4 and 0.1 M NaOH were used for pH adjustment. In Al2(SO4)3 experiments, 64.8, 32.4, 16.2, 8.1, 4.05, and 0 mg/L were added to each beaker and studied separately at pH 3, 5, 7, 9, and 11. C-FeCl3 and MS-FeCl3 experiments were also carried out by adding the coagulant at 20.64, 10.32, 5,16, 2.58, 1.29, and 0 mg/L concentrations at the same variable pH values. In all experiments, samples were collected taken from approximately 2 cm below the water surface with the help of a sterile syringe without moving the flocks that had sunk to the bottom. The pH value with the highest efficiency regarding the COD removal parameter of all three coagulants was selected as the optimum, and the second stage was started according to these values. By keeping the optimum pH and coagulant dosages determined at this stage constant, the optimum flocculant dosage was determined based on with variable flocculant dosages (4, 2, 1, 0.5, 0.25, and 0 mg/L).
Results and discussion
MS-FeCl3> qualification
Effect of HCl concentration on FeCl3 production from MS
Two methods are commonly used in the production of FeCl3. The first method is the production of FeCl3 through the reaction of scrap iron with chlorine gas. The second and more widely used method is the production of FeCl3 from dirty acid solutions coming out of the surface cleaning line (pickling line) with HCl during iron-steel production. In general, H2SO4 is used as an acid in the surface cleaning process. However, recently, the use of HCl has become increasingly widespread. If scale is used as an alternative to ferrous scrap, the production process remains the same as for scrap iron. Additionally, the high iron content of scale and its ability to be ground are advantages in FeCl3 production and reaction kinetics. Fe is found in MS at various oxidation steps. Due to its high potential, it is desired that the iron found in the solution as a result of treating the iron in the MS with HCl be mostly in the form of Fe3+. Here, it is essential to convert the iron in the MS into various forms of oxidizing ability (Fe3+ = + 0.771 V/Fe2+ = + 0.440 V) in the solution medium and use it in the wastewater of I&SI. The reactions occurring during the formation of FeCl3/FeCl2 from the MS were as follows:
Furthermore, in the solution with an oxidizing character to be obtained from MS, in addition to the solubility of iron, the rate of its conversion into Fe3+ is also important. Depending on the HCl concentration, the Cl2 gas generated during the leaching process would remain in the pressure-resistant sealed glass tubes where the test is performed. This situation will also significantly contribute to the conversion of some FeCl2 into FeCl3 as described in Eq. (8). In this context, FeCl3/FeCl2 production from MS in the presence of HCl was investigated under the conditions of various leaching parameters.
The effect of acid concentration was investigated at 1–9 M HCl concentrations at a constant leaching temperature for 378 K 120 min. Figure 2 shows the effect of acid concentration on Fe3+ conversion in the dissolution of iron from MS. The rate of Fe3+ concentration was increased by increasing the acid concentration. Fe3+ conversion in the solution was initially low but it increased at high concentrations. In the leaching conditions with a leaching temperature of 378 K, a solid–liquid ratio of 1/10 (g/mL), leaching times of 120 min, and HCl concentration of 7 M, it was seen that the ratio of iron taken into the solution was 100%. At the same time, the Fe3+ conversion rate was approximately 77%. The results of previous studies (Schwertmann and Taylor 2018) showed that iron oxide dissolution was maximized with HCl solutions between 6 and 12 mol/L. The higher dissolution in HCl was due to the oxidation power of chloride ions. Additionally, the acid concentration is essential for economic concerns.
Effect of reaction temperature and time on FeCl3 production from MS
Figure 3 shows the effect of contact time on Fe3+ conversion as a function of temperature. The Fe3+ concentration was increased rapidly with the increase leaching temperature and time. The experiment that was carried out at a temperature of 318 K, for 180 min, at a 7 M HCl concentration and a 1/10 (g/mL) solid–liquid ratio, achieved a 25.9% Fe3+ conversion rate. Likewise, at a temperature of 378 K, all iron was taken into the solution, and a Fe3+ conversion rate an approximately 80% was observed. Here, the increasing dissolution rate was due to the continued oxidation of metal atoms into metal ions until saturation. Because the iron passing into the solution during the treatment of MS with HCl would be in the forms of both Fe3+ and Fe3+, it was aimed to identify the conditions with the highest Fe3+ conversion rate. Based on this issue, the optimum conditions were determined as a 378 K leaching temperature, a reaction time of 120 min, a solid–liquid ratio of 1/10 g/mL, an HCl concentration of 7 M, and a particle size of − 90 μm. The rate of dissolved Fe was 100% in these conditions, while the Fe3+conversion rate and concentration were approximately 77% and 52.53 g/L, respectively. The solutions obtained in these conditions were stored to treat the I&SI wastewater.
Moreover, to understand the dissolution behavior of iron from MS under thermoreactor conditions and different temperatures with representative parameters, the Eh–pH diagrams of the possible compounds belonging to the Fe-HCl-H2O system were created (Fig. 4). Eh–pH diagrams for the Fe-HCl-H2O system were drawn using the HSC/EpH—Eh–pH diagram module. Eh–pH diagrams also offer a quick way of studying the dissolution behavior of different materials. Using the thermodynamic data of the reactions that multiple metals could show in the aqueous medium, the Eh–pH diagram was formed by combining the dissolvability values of oxides and hydroxides and the equilibrium constants of the reactions (Turan et al. 2022). Accordingly, it was understood that FeCl3/FeCl2 ion species could only be obtained with a decrease in pH and an increase in Eh in the reaction of MS with HCl under the current conditions. However, by the increase in the temperature of the medium, the presence of FeCl3 shifted to a broad region. FeCl3 was replaced ferric oxyhydroxide (FeO*OH) at high Eh values and all pH values.
Characterization of MS leaching solution and leaching residue
At the optimum leaching conditions (leaching temperature of 378 K, leaching time of 120 min, a solid–liquid ratio of 1/10 g/mL, HCl concentration of 7 M), ICP, Raman spectroscopy, and zeta potential measurements to determine the isoelectric point, respectively, in the MS leaching solution were carried out. The results of the ICP analysis of the MS leaching solution obtained under the optimum conditions are shown in Table 3. As seen in Table 3, all elements, except for iron, which was found the scale and completely dissolved, dissolved at specific rates. However, it was determined that their dissolution rates were far below the chemical composition values of facilities that produce FeCl3 at the national and international scales.
The MS aqueous solution samples at the optimum conditions were analyzed by Raman microspectroscopy (WITEc—Alpha M +). Analyses were also carried out with a 532 nm (argon) laser. The Raman signal was collected with a spectral resolution of 1 cm−1 over 100–4000 cm−1 using × 20 or × 50 microscope lenses. Raman spectroscopy is a non-destructive chemical analysis technique that provides detailed information about chemical structure, phase and polymorphism, crystallinity, and molecular interactions. It is based on the interaction of light with the chemical bonds within a material. Raman spectroscopy is a sensitive method to characterize different Fe2+ and Fe3+ species structures in aqueous solutions. The results of the Raman analyses of the leaching solution obtained under the optimum leaching conditions indicating different phases are shown in Fig. 5. The Raman analysis showed the coordination of Fe3+ and Fe2+ with Cl−.
The analysis of the Raman spectra indicated that the peak at ~ 332 cm−1 corresponded to FeCl3 and FeCl4 ([Fe(H2O)6]3+ + 4Cl− = [FeCl4]− + 6H2O); the peak at 414 cm−1 corresponded to Fe2Cl6; the peak at 1413 cm−1 corresponded to Fe3+-OH2; the peaks at 1314, 1572, and 3412 cm−1 corresponded FeCl2; and the peak at 2800–3800 cm−1 corresponded to H2O/OH vibrational mode (Givan and Loewenschuss 1977; Voyiatzis et al. 1999; Baumgartner 2009; Kong et al. 2011; Takaya et al. 2019; Zeng et al. 2020). The two peaks observed below 200 cm−1 were the characteristic Raman bands common to all halide ion solutions, which arise from the translational vibrations of water molecules that are hydrogen-bonded to either halide ions or other water molecules (Kanno and Hiraishi 1982).
Zeta potential measurements were made with a Zetasizer-Malvern (Nano ZS90) device at room temperature to determine the isoelectric point of the MS aqueous solution sample under the optimum conditions. The pH at the point where the zeta potential is zero is defined as the isoelectric point (pip). The isoelectric point is where the system is the most unstable. In the analyses conducted accordingly, it was determined that the isoelectric point was in the acidic region (pH:3.1). The zeta potential also became more negative as the pH of the solution increased.
Batch wastewater treatment results
Determination of optimum pH, coagulant dosages, and flocculant dosages
pH is a significant factor in the coagulation process. Each coagulant has a pH value at which it is active. A small change in pH can stabilize or destabilize dispersions. Pollutants in dissolved or colloidal form in water are subject to chemical surface changes in every pH range (López-Maldonado et al. 2014). When a metal is added to water at a certain pH, dissolution occurs. Metal (M) ions present in water are hydrolyzed to form monomeric, polymeric, and solid precipitates as follows: M(OH)2+, MOH2+, M2(OH)24+, M(OH)45+, M(OH)30(s), and M(OH)4− (Dentel and Gossett 1988; Verma et al. 2010). In particular when Fe salts are added, the pH value decreases. The metal precipitates from the optimum pH range of 3–6 (Eq. 9) (Verma et al. 2010). Therefore, pH needs to be adjusted for the optimum efficiency (Amuda and Amoo 2007; Qiao et al. 2012).
Additionally, a decrease in pH appears with the addition of aluminum salts, which reduces the efficiency of precipitation. The optimum precipitation efficiency was observed in the pH range of 5.7–7 (Eq. 10) (Benjamin 2014).
To determine the optimum pH effect in the performance trials of the Al2(SO4)3, C-FeCl3, and MS-FeCl3 coagulants, a fixed anionic polyelectrolyte dosage (1.0 mg/L) was first studied. Long-chain polymeric flocculants attract particles formed with metal salts by adsorption, and they are used to ensure the faster precipitation of these particles by increasing their size and weight (Wei et al. 2018). As the effect of precipitation time on precipitation efficiency was negligible as a result of the preliminary experiments, the precipitation time variable was not studied (Dotto et al. 2019). The COD, TSS, color, and turbidity removal efficiencies at variable pH values in the removal studies using the aforementioned coagulants are presented in Fig. 6.
In the process outlined in Fig. 6-A, the dosage of Al2(SO4)3 as the coagulant was kept in the range of 0–16 mg/L, and 1.0 mg/L anionic polyelectrolyte was added as a flocculant. As seen in Fig. 7, in the removal study with Al2(SO4)3, the most effective pH in COD removal was 7.0, although there was still removal of turbidity, color, and TSS, except for pH 7.0, below and above the neutral among the different pH values. On the other hand, as the dosage of Al2(SO4)3 increased, it is clearly seen that the yield did not increase in every parameter, but decreased. When the Al2(SO4)3 dosage was increased from 0 to 4.05 mg/L at pH 7.0, the removal efficiency of COD was around 50%, but when the dosage was 8.1 mg/L, the efficiency rose to around 70%. Nevertheless, it can be easily seen that the efficiency value did not increase when the Al2(SO4)3 dosage was increased. Therefore, it was unnecessary to dose more Al2(SO4)3. In summary in the presence of 4 mg/L Al2(SO4)3, turbidity, color, and TSS removal increased above 80% at almost every pH value, while COD removal efficiency was calculated as 68.52% in the presence of 4 mg/L Al2(SO4)3 at pH 7.0. Using dosages in the range of, after 0–22 mg/L C-FeCl3 at variable pH values (Fig. 6-B), 1.0 mg/L anionic polyelectrolyte was added again. In the removal study with C-FeCl3, turbidity, color, and TSS removal was observed, except for pH 5.0, but it can be seen that this was the most effective pH value for COD removal. It can be seen that the removal efficiency of COD reached around 70% when the C-FeCl3 dosage is increased from 0 to 1.29 mg/L at this pH, but the efficiency decreased when the dosage was increased further. Therefore, it is clear that further dosing of C-FeCl3 was also unnecessary. Accordingly, at low C-FeCl3 dosages (below 2 mg/L), the removal efficiencies of turbidity and color were high than 70%, while the yield tended to decrease when the dosage was increased. On the other hand, it was clear that the removal efficiencies were high at pH 11. However, this is due to the precipitation of calcium ions naturally found in water as CaCO3 at high pH, as well as the precipitation of magnesium ions as Mg(OH)2 (Semerjian and Ayoub 2003). Therefore, the COD removal efficiency was calculated as 64.66% in the presence of 0.0125 mg/L C-FeCl3. In the coagulation experiments with MS-FeCl3 produced from the MS examined, the exact dosages of C-FeCl3 were applied. The plot curves were found to be very similar to the curves in the C-FeCl3 studies, as seen in Fig. 6. In the removal study with MS-FeCl3, as in the C-FeCl3 dosage studies, removal was observed in 3 parameters except pH 5.0. Considering the COD parameter, on the other hand, it can be seen that the most effective pH was again 5.0. Again, it was seen that the removal efficiency of COD reached around 70% when the MS-FeCl3 dosage increased from 0 to 1.29 mg/L at this pH. However, it was clearly seen that the efficiency decreased when the dosage was increased further. Therefore, further dosing was unnecessary in the MS-FeCl3 study as it was in the C-FeCl3 study. In summary, the COD removal efficiency was calculated as 66.59% in the presence of 0–22 mg/L MS-FeCl3 (in Fig. 6-C). Moreover, the optimum conditions for the highest COD removal when Al2(SO4)3 was used as the coagulant was 4.05 mg/L at pH 7.00 among the varied pH values. The optimum conditions for the use of both the C-FeCl3 and MS-FeCl3 coagulants were a dose of 1.29 mg/L and a pH value of 5.00. The optimum pH and dosage value amounts were found by evaluating the removal efficiencies of all three coagulants based on COD. By keeping these values constant, optimum anionic polyelectrolyte content experiments were carried out.
In Fig. 7, it is seen that the anionic polyelectrolyte dosage was kept in the range of 0–4 mg/L at 4.05 mg/L Al2(SO4)3 and at pH 7.0. As the dosage increased up to 2.0 mg/L, the yield also increased, but the yield decreased when the dosage was raised further. The COD, TSS, color, and turbidity removal efficiencies at 2.0 mg/L of polyelectrolyte were calculated as 70.46, 87.13, 85.06, and 78.57%, respectively. It was observed that the efficiency decreased when the anionic polyelectrolyte dosage was increased to the dosage of 1.29 mg/L C-FeCl3 at pH 5.0, and the best efficiency was obtained at the dosage of 0.25 mg/L polyelectrolyte. In these dosages, the COD, TSS, color, and turbidity removal efficiencies were calculated as 68.52, 81.85, 71.22, and 82.96%, respectively. Additionally, in the MS-FeCl3 study, a situation similar to the C-FeCl3 study emerged, and the COD, TSS, color, and turbidity removal efficiencies in the presence of 1.29 mg/L MS -FeCl3 and 0.25 mg/L polyelectrolyte dosage were 57.53, 91.53, 89.05, and 83.55, respectively. In the removal mechanism examined, trivalent aluminum and iron cations, which are metal salts entering the aqueous medium, had very similar characteristics. When these salts are dissolved in water, hydrates of metal (M) ions are formed and hydrolyzed to monomeric or polymeric species (MOH+2, M(OH)2+, M(OH)24+, M(OH)4+5, M(OH)3, and M(OH)4−). Under acidic conditions, these salts are in the solution, but when the pH is increased or the concentrations of these salts increased, ferric hydroxide (Fe(OH)3(s)) or aluminum hydroxide (AI(OH)3(s)) forms are created. In other words, all metals have the minimum solubility if there are enough coagulants in the environment and at a pH where there is minimal solubility. Likewise, these create hydroxide forms with a gelatinous appearance. These forms are amorphous, they are hydrophobic, have a large surface area, and are positively charged. Since pollutants in the water generally have a negative charge, they are adsorbed on the surfaces of these formed metal particles and become insoluble. Immediately after this process, when precipitation occurs, pollutants are removed (Stephenson and Duff 1996; Pang et al. 2009; Bratby 2016). As a result of evaluating all three coagulants based on their optimum COD removal efficiency values, the optimum pH and dosage values were determined, and optimum anionic polyelectrolyte were found by carrying out experiments while keeping these values constant.
Heavy metal removal efficiencies and toxicological assessment under optimum conditions
The results of the analyses of the effluents obtained at the end of the jar tests with the three coagulants and the results of the analyses of the raw wastewater are presented in Table 4. In the raw wastewater, Zn, Fe, Mn, and Pb concentrations were high. As a result of the treatment studies with Al2(SO4)3, more than 96.6% efficiency was obtained in the removal of other metals. The treatment results with C-FeCl3 showed a removal efficiency of over 94.6%. The treatment studies with MS-FeCl3 achieved a treatment efficiency of over 94.5%. Again, the heavy metal removal mechanism acted as a general precipitation mechanism. The visual representation of this mechanism is presented in Fig. 8. That is, at the optimum pH where Fe(OH)3(s) and Al(OH)3(s) ions, which become polymerized and have a high surface area, do not dissolve, heavy metals are adsorbed onto these hydroxides and precipitate out together (Pang et al. 2009; Dubery et al. 2017).
In the examinations regarding Zn, which is one of the primary metal pollutants, the removal efficiency was found to be 98.91% at the dosage of 4.05 mg/L Al2(SO4)3 and 2.0 mg/L anionic polyelectrolyte. Following the same amount (1.29 mg/L) of C-FeCl3 and the same MS-FeCl3 dosage, the removal efficiencies of Zn were calculated as 94.60% and 97.14%, respectively, at the 2.0 mg/L anionic polyelectrolyte dosage. Furthermore, the removal efficiency of Fe was over 99.99% in the case of the addition of Al2(SO4)3 at the aforementioned doses. In comparison, 99.53% and 99.79% efficiency values were obtained by the same dosages of C-FeCl3 and MS-FeCl3, respectively. Additionally, the highest Pb removal efficiency was seen in the case of using MS-FeCl3 as 99.99%. Again, the removal efficiency of Mn using MS-FeCl3 was calculated as 98.46%, while the removal efficiencies obtained when Al2(SO4)3 and C-FeCl3 were used were 98.74% and 93.56%, respectively. If a general evaluation is made in terms of heavy metal removal efficiencies, these efficiencies were very close to each other at the same value (2.0 mg/L) of anionic polyelectrolyte dosage, with the same dosages (1.29 mg/L) of C-FeCl3 and MS-FeCl3.
As it is known, heavy metals have high atomic weight. They also have a specific gravity at least 5 times greater than the specific gravity of water. Toxic metals, including heavy metals, are metal compounds that have a negative impact on human and environmental health. The toxicities of these metals depend on dosage, type of meat, and exposure. It also depends on the specific characteristics of the exposed creature (age, gender, genetic factors, and feeding habits). Most known metals, especially heavy metals, are toxic, but some are essential and some, such as bismuth, are slightly less toxic than others (Tchounwou et al. 2012). On the other hand, metals with abnormal oxidation can also become toxic: For example, chromium (III) is an essential trace element, but chromium (VI) is known to be carcinogenic. Moreover, compounds containing soluble metals show toxic properties. Soluble metals are called coordination complexes consisting of a metal ion surrounded by ligands (Ali et al. 2013).
Heavy metals act toxically after a certain concentration (1–10 ppm) (Fe, Cu, Zn, Ni, and Se) (Järup 2003). On the other hand, this concentration value varies depending on the presence of the heavy metal in soil and water. These limit values for some metals in soil are given by the World Health Organization (WHO): Ni 80, Cu 30, Cr 100, Mn 200, As 20, Sb 36, Fe 50,000, and Zn 300 ppm (WHO 1996). In this study, as seen in Table 3, the ratio of some metals found in the leach solution and included in the heavy metal class is given. It has been determined that at the end of the treatment, some or all of these metals precipitate and remain in the sewage sludge, and these metals do not have any toxic effects compared to the rates that can be found in the soil, according to the WHO. On the other hand, according to the WHO, the maximum acceptable concentrations of some toxic metals in drinking water for copper, nickel, arsenic, antimony, manganese, zinc, aluminum, mercury, lead, iron, and chromium are given as 2, 0.07, 0.01, 0.02, 0.05, 3, 0.2, 0.006, 0.01, 3, and 0.05 mg/L respectively (WHO 2022). As a result of the jar test performed with MS-FeCl3 in this study, it shows that the toxic metal rates in the treated wastewater are below acceptable values, as seen in Table 4. This proves that treated wastewater has no toxicity effect according to the WHO.
Sludge characterization
The SEM images that display the morphological characteristics of the sludge, the elemental composition of the sludge based on weight (%), and the EDX spectra of the sludge obtained at optimum operating conditions using Al2(SO4)3, C-FeCl3, and MS-FeCl3 as coagulants are presented in Fig. 9 (a–c). The SEM images of the samples showed lumpy particles with an average size of several micrometers for different coagulants. The SEM images indicated that the solids had different forms and sizes and connected they formed flocs. The EDX spectra of the sludge obtained by using Al2(SO4)3, C-FeCl3, and MS-FeCl3 as coagulants showed the presence of Zn, Ca, Mg, Na, and Si in the sludge. Since some steel production operations are carried out using zinc-coated (galvanized) scraps, the zinc ion density in the plant’s wastewater was also high. These zinc ions were successfully precipitated, as seen in the elemental composition of the sludges. Additionally, it is thought that the element Si in the sludges come from the ore and raw materials used for slag-making during the production of raw iron. The carbon detected in the sludge corresponded to the carbon planchet used for the SEM micrographs, and the gold found in the sludge was associated with the gold coating used for the SEM process. Carbon and gold were not included in the total weight percentage calculations. When these elements were added, the cumulative percentage by weight become 100%. The results of the EDX analysis suggested that this procedure successfully recovered a substantial part of the inorganic species from the I&SI wastewater. A flowsheet mechanism of the processes designed for this study is illustrated in Fig. 10.
Comparison of results to literature
Information about some studies in which the removal of pollutants from iron-steel industry wastewater was carried out using the coagulation-flocculation method is presented in Table 5. In a previous study, in the steel production factory, the cooling liquid of the hot cylinder was treated, where Al2(SO4)3, FeCl3, and FeSO4 were used as coagulants at dosages ranging from 1.0 to 3.0 mg/L. Anionic, nonionic, and cationic polyelectrolytes were used as flocculants at concentrations of 4.0 mg/L. These coagulants were found to be quite efficient, and it was seen that they removed more than 98% of the TSS. Additionally, while the turbidity unit of the wastewater was 20 NTU, the turbidity decreased below 5 NTU after the removal processes carried out at the optimum dosages of Al2(SO4)3, FeCl3, and FeSO4. The authors emphasized that the addition of flocculant increased the efficiency of the treatment process (Kim et al. 2010). Similarly, in a study where automobile coating wastewater was treated using Al2(SO4)3 and CaO with the same method, it was reported that the removal efficiency was 97.1% in the turbidity parameter and 10.5% in the COD parameter (Xiong et al. 2017). In another study, synthetic and actual electroplating industry wastewater, which included heavy metals, was treated for the removal of heavy metals using FeCl3 at the coagulant, and it was reported that the resulting product met the effluent standards in terms of heavy metals (Puasa et al. 2021). In this study, success was achieved in the removal of organic and inorganic pollutants by conducting coagulation and flocculation experiments with commercial Al2(SO4)3 and C-FeCl3 as well as MS-FeCl3 obtained from a waste product namely MS in actual I&SI wastewater. The polyelectrolyte dosages used in the jar tests had similar effects on COD, TSS, color, and turbidity removal in all three settings with the aforementioned coagulants. When MS-FeCl3 and C-FeCl3 were used at the same dosages (1.29 mg/L), the COD removal efficiency result was around 60% at the same polyelectrolyte dosage (0.25 mg/L), whereas other parameters were similar at removal efficiency values around 85–90%.
Scale-up potential and future perspectives for FeCl3 production and wastewater treatment
The coagulation-flocculation method is the most common treatment method used in the treatment of large-scale industrial wastewater. Its high efficiency, ease of operation, and low maintenance cost make this method popular today compared to other systems (Teh et al. 2016). It is convenient for treating industrial wastewaters originating from fields such as the textile, mining, metallurgy, chemistry, and food industries (Zhao et al. 2021; Tran et al. 2023). Coagulants such as Al2(SO4)3, FeCl3, Fe2(SO4)3, PAC, and PAFC are used in the implementation of the process (Verma et al. 2012). The production of these chemicals requires highly complex processes, and it is known to have high costs. While market prices vary day by day, the price of FeCl3 is approximately 0.30 US$/kg, and the price of Al2(SO4)3 is around 0.20 US$ /kg. Considering the amount of Al2(SO4)3 and FeCl3 consumed per unit m3 of our wastewater, figures are around 1.013 US$ for Al2(SO4)3 and 0.387 US$ for FeCl3 (excluding taxes and other technical equipment). On the other hand, MS, which originates and emerges from currently used iron-steel production processes and is an intermediate product, can be used as an alternative, thanks to the process we propose. This is because this way, a product which is considered waste would be returned to the economy. Additionally, a more straightforward and more accessible production method was developed in this study rather than a method involving complex processes. The stoichiometric proof of FeCl3 production from sludge was provided, and the feasibility of this process was demonstrated by analyses on the contents of metals, as well as elemental pollutants by conducting treatment studies of the wastewater of an actual facility. There was no difference between the operation of the rapid mixing ponds during the functioning of the wastewater treatment plant compared to the commercial product. Considering the market share of FeCl3 in the world, it is evident that the proposed process in the treatment of wastewater with high flow rates will be beneficial both in terms of carbon footprint and economically. On the other hand, the use of coagulants that pass into the sludge as a result of the treatment process should also be evaluated. The amount of acid consumed during recovery also affects the total cost. Additionally, alternative treatment methods for the neutralization and removal of other pollutants are very important due to the acidic character of the wastewater generated during recycling and the fact that these wastewaters may contain heavy metals or organic/inorganic pollutants (Xu et al. 2009; Chakraborty et al. 2020).
Conclusion
This study investigated the treatment of MS, which has a high iron content and is a significant waste of the iron-steel industry, in the presence of HCl and usage conditions of the obtained solution in the treatment of iron and steel wastewater. The results are summarized as follows:
-
In the experiments where MS was treated with HCl and under the optimum conditions (7 M HCl, leaching temperature 378 K, leaching time 120 min, solid–liquid ratio 1/10 (g/mL), particle size − 90 μm), 100% Fe solubility, approximately 77% Fe3+ conversion rate, and 52.53 g/L Fe3+ concentration were achieved.
-
Turbidity, color, and TSS removal efficiency values were found to be above 80%, while COD removal efficiency was 68.52% at the dosage of 4.05 mg/L Al2(SO4)3 and 2.0 mg/L anionic polyelectrolyte.
-
Both MS-FeCl3 and C-FeCl3 were applied at a dosage of 1.29 mg/L. The turbidity, color, and TSS removal efficiency values were above 70% at the 0.25 mg/L anionic polyelectrolyte dosage. The COD removal efficiency value for MS-FeCl3 was 66.59%, while this was 65.66% with C-FeCl3.
-
Heavy metal removal efficiency values over 96% were obtained in the Al2(SO4)3 study in terms of Fe, Cr, Mn, Ni, Zn, Cd, Hg, and Pb, which were also examined under the optimum conditions. In the MS-FeCl3 and C- FeCl3 studies, a removal efficiency of over 94.50% was achieved.
Consequently, the results of this study showed that the treatment performance of MS-FeCl3 obtained from MS was higher than that of Al2(SO4)3 and similar to that of C-FeCl3. The results of the heavy metal analyses also showed that with MS-FeCl3 obtained from MS, one of the wastes of the iron and steel industry, the wastewater of this sector was treated, contributing to sustainable waste management. Furthermore, this method is considered to be important in terms of economic and environmental awareness to dispose of a waste, obtain a coagulant from this waste, and use this coagulant successfully in a of wastewater treatment process.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Abou-Elela SI, Fawzy ME, El-Shafai SA (2021) Treatment of hazardous wastewater generated from metal finishing and electro-coating industry via self-coagulation: case study. Water Environ Res 93:1476–1486. https://doi.org/10.1002/WER.1552
Abujazar MSS, Karaağaç SU, Abu Amr SS et al (2022a) Recent advancement in the application of hybrid coagulants in coagulation-flocculation of wastewater: a review. J Clean Prod 345:131133. https://doi.org/10.1016/J.JCLEPRO.2022.131133
Abujazar MSS, Karaağaç SU, Amr SSA et al (2022b) The effectiveness of rosehip seeds powder as a plant-based natural coagulant for sustainable treatment of steel industries wastewater. Desalination Water Treat 270:44–51. https://doi.org/10.5004/DWT.2022.28782
Ahmaruzzaman M (2011) Industrial wastes as low-cost potential adsorbents for the treatment of wastewater laden with heavy metals. Adv Colloid Interface Sci 166:36–59. https://doi.org/10.1016/J.CIS.2011.04.005
Akbal F, Camci S (2010) Comparison of electrocoagulation and chemical coagulation for heavy metal removal. Chem Eng Technol 33:1655–1664. https://doi.org/10.1002/CEAT.201000091
Ali H, Khan E, Sajad MA (2013) Phytoremediation of heavy metals—concepts and applications. Chemosphere 91:869–881. https://doi.org/10.1016/j.chemosphere.2013.01.075
Al-Shannag M, Al-Qodah Z, Bani-Melhem K et al (2015) Heavy metal ions removal from metal plating wastewater using electrocoagulation: kinetic study and process performance. Chem Eng J 260:749–756. https://doi.org/10.1016/J.CEJ.2014.09.035
Altair Chimica (2018) Cloruro Ferrico Standard (UNI EN 888). https://www.altairchimica.com/en/products/ferric-and-ferrous-chloride/. Accessed 3 Jun 2023
Amuda OS, Amoo IA (2007) Coagulation/flocculation process and sludge conditioning in beverage industrial wastewater treatment. J Hazard Mater 141:778–783. https://doi.org/10.1016/J.JHAZMAT.2006.07.044
AWWA WA (2017) Standard methods for the examination of water and wastewater, 23rd edn. American Public Health Association, Washington, DC
Baghel R, Pandel U, Vashistha A (2020) Manufacturing of sustainable bricks: utilization of mill scale and marble slurry. Mater Today Proc 26:2136–2139. https://doi.org/10.1016/J.MATPR.2020.02.460
Bantsis G, Betsiou M, Bourliva A et al (2012) Synthesis of porous iron oxide ceramics using Greek wooden templates and mill scale waste for EMI applications. Ceram Int 38:721–729. https://doi.org/10.1016/J.CERAMINT.2011.07.064
Baryshev VG, Gorelov AM, Folders GI (2011) Secondary material resources of ferrous metallurgy: a reference book. M: Economics
Baumgartner M (2009) Analysis of salt-bearing aqueous solutions in synthetic fluid inclusions by microthermometry and cryogenic Raman spectroscopy. University of Leoben
Beauchemin D (2008) Inductively coupled plasma mass spectrometry. Anal Chem 80:4455–4486. https://doi.org/10.1021/AC8006945/ASSET/AC8006945.FP.PNG_V03
Beh CL, Chuah TG, Nourouzi MN, Choong T (2012) Removal of heavy metals from steel making waste water by using electric arc furnace slag. E-J Chem 9:2557–2564
Benjamin MM (2014) Water chemistry, 2nd edn. Waveland Press, Ottawa
Biswas J (2013) Evaluation of various method and efficiencies for treatment of effluent from iron and steel industry-a review. Int J Mech Eng Robot Res 2:67–73
Bratby J (2016) Coagulation and flocculation in water and wastewater treatment. IWA Publishing, IWA Publishing
Chakraborty T, Balusani D, Smith S et al (2020) Reusability of recovered iron coagulant from primary municipal sludge and its impact on chemically enhanced primary treatment. Sep Purif Technol 231:115894. https://doi.org/10.1016/j.seppur.2019.115894
Changmai M, Mondal P, Sinha A et al (2020) Metal removal efficiency of novel LD-slag-incorporated ceramic membrane from steel plant wastewater. Int J Environ Anal Chem 102:1078–1094. https://doi.org/10.1080/03067319.2020.1734198
Colla V, Branca TA, Rosito F et al (2016) Sustainable Reverse Osmosis application for wastewater treatment in the steel industry. J Clean Prod 130:103–115. https://doi.org/10.1016/J.JCLEPRO.2015.09.025
Das P, Mondal GC, Singh S et al (2018) Effluent treatment technologies in the iron and steel industry - a state of the art review. Water Environ Res 90:395–408. https://doi.org/10.2175/106143017X15131012152951
Dentel SK, Gossett JM (1988) Mechanisms of coagulation with aluminum salts. J Am Water Works Assoc 80:187–198. https://doi.org/10.1002/J.1551-8833.1988.TB03025.X
Dotto J, Fagundes-Klen MR, Veit MT et al (2019) Performance of different coagulants in the coagulation/flocculation process of textile wastewater. J Clean Prod 208:656–665. https://doi.org/10.1016/J.JCLEPRO.2018.10.112
Dubery S, Agarwal M, Gupta AB (2017) A study on the characterisation of the species formed during fluoride removal through coagulation. Interdiscip Environ Rev 18:143. https://doi.org/10.1504/IER.2017.087914
El-Hussiny NA, Abdul-Wahab HH, Ali MM et al (2014) Effect of grinding time of mill scale on the physicochemical properties of produced briquettes and its reduction via hydrogen. Open Access Libr J 1:1–10
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manage 92:407–418. https://doi.org/10.1016/J.JENVMAN.2010.11.011
Garg R, Singh SK (2022) Treatment technologies for sustainable management of wastewater from iron and steel industry — a review. Environ Sci Pollut Res 29(50):75203–75222. https://doi.org/10.1007/S11356-022-23051-3
Givan A, Loewenschuss A (1977) Matrix isolation Raman spectra of FeCl3 and Fe2Cl6. J Raman Spectrosc 6:84–88. https://doi.org/10.1002/JRS.1250060207
Gülensoy H (1968) Kompleksometrinin Esasları ve Kompleksometrik Titrasyonlar, 4th edn. Fatih Yayınevi Matbaası, İstanbul
Gündoğdu N (2013) Demir-Çelik Tesislerinde Açığa Çıkan Tufalden Demirin Geri Kazanımı. İstanbul Teknik Üniversitesi
Iftikhar R, Parveen I, Ayesha et al (2023) Small organic molecules as fluorescent sensors for the detection of highly toxic heavy metal cations in portable water. J Environ Chem Eng 11:109030. https://doi.org/10.1016/j.jece.2022.109030
Iluiu-Varvara DA, Aciu C, Tintelecan M, Sas-Boca IM (2020) Assessment of recycling potential of the steel mill scale in the composition of mortars for sustainable manufacturing. Procedia Manuf 46:131–135. https://doi.org/10.1016/J.PROMFG.2020.03.020
Järup L (2003) Hazards of heavy metal contamination. Br Med Bull 68:167–182. https://doi.org/10.1093/bmb/ldg032
Jikar PC, Dhokey NB (2021) Overview on production of reduced iron powder from mill scale waste. Mater Today Proc 44:4324–4329. https://doi.org/10.1016/J.MATPR.2020.10.552
Jung YJ, Kiso Y, Yamada T et al (2006) Chemical cleaning of reverse osmosis membranes used for treating wastewater from a rolling mill process. Desalination 190:181–188. https://doi.org/10.1016/J.DESAL.2005.08.009
Kanno H, Hiraishi J (1982) A Raman study of aqueous solutions of ferric nitrate, ferrous chloride and ferric chloride in the glassy state. J Raman Spectrosc 12:224–227. https://doi.org/10.1002/JRS.1250120305
Karam A, Bakhoum ES, Zaher K (2020) Coagulation/flocculation process for textile mill effluent treatment: experimental and numerical perspectives. 14:983–995.https://doi.org/10.1080/19397038.2020.1842547
Khaerudini DS, Chanif I, Insiyanda DR et al (2019) Preparation and characterization of mill scale industrial waste reduced by biomass-based carbon. J Sustain Metall 5:510–518. https://doi.org/10.1007/s40831-019-00241-x
Khan MH, Ha DH, Jung J (2014) Metal etching industry wastewater pretreatment by coagulation and ion exchange resins. Desalination Water Treat 52:2433–2439. https://doi.org/10.1080/19443994.2013.794712
Kim TH, Ha DW, Kwon JM et al (2010) Purification of the coolant for hot roller by superconducting magnetic separation. IEEE Trans Appl Supercond 20:965–968. https://doi.org/10.1109/TASC.2010.2043521
Kong WG, Wang A, Freeman JJ, Sobron P (2011) A comprehensive spectroscopic study of synthetic Fe2+, Fe 3+, Mg2+ and Al3+ copiapite by Raman, XRD, LIBS, MIR and vis-NIR. J Raman Spectrosc 42:1120–1129. https://doi.org/10.1002/JRS.2790
Koruma Group Companies (2019) Ferric chloride solution type I. Kocaeli/Turkey
Li L, Fan M, Brown RC et al (2009) Production of a new wastewater treatment coagulant from fly ash with concomitant flue gas scrubbing. J Hazard Mater 162:1430–1437. https://doi.org/10.1016/J.JHAZMAT.2008.06.035
Lisin VS, Skorokhodov VN, Kurunov IF et al (2003) Resource and ecological solutions for disposal of wastes from metallurgical production. Ferrous Metall 10:64
López-Maldonado EA, Oropeza-Guzman MT, Jurado-Baizaval JL, Ochoa-Terán A (2014) Coagulation–flocculation mechanisms in wastewater treatment plants through zeta potential measurements. J Hazard Mater 279:1–10. https://doi.org/10.1016/j.jhazmat.2014.06.025
Mahjouri M, Ishak MB, Torabian A et al (2017) Optimal selection of Iron and Steel wastewater treatment technology using integrated multi-criteria decision-making techniques and fuzzy logic. Process Saf Environ Prot 107:54–68. https://doi.org/10.1016/j.psep.2017.01.016
Mainardis M, Mulloni S, Catenacci A et al (2022) Sustainable alternatives for tertiary treatment of pulp and paper wastewater. Sustainability 14:6047. https://doi.org/10.3390/SU14106047
Mondal M, Mukherjee R, Sinha A et al (2019) Removal of cyanide from steel plant effluent using coke breeze, a waste product of steel industry. J Water Process Eng 28:135–143. https://doi.org/10.1016/j.jwpe.2019.01.013
Oden MK, Sari-Erkan H (2018) Treatment of metal plating wastewater using iron electrode by electrocoagulation process: optimization and process performance. Process Saf Environ Prot 119:207–217. https://doi.org/10.1016/J.PSEP.2018.08.001
Önkibar G (2006) Entegre demir-çelik tesisi tufalinden doğrudan redüklenme yöntemi ile ham demir üretimi. Sakarya Üniversitesi
Osman I (2012) Tufalin demir cevheri konsantresi ile karıştırılarak pelet üretiminde kullanılabilirliğinin ve indirgenebilirliğinin incelenmesi. İstanbul Teknik Üniversitesi
Ozturk M, Depci T, Bahceci E et al (2020) Production of new electromagnetic wave shielder mortar using waste mill scales. Constr Build Mater 242:118028. https://doi.org/10.1016/J.CONBUILDMAT.2020.118028
Pang FM, Teng SP, Teng TT, Mohd Omar AK (2009) Heavy metals removal by hydroxide precipitation and coagulation-flocculation methods from aqueous solutions. Water Qual Res J 44:174–182. https://doi.org/10.2166/WQRJ.2009.019
Pang AL, Arsad A, Ahmad Zaini MA et al (2023) A comprehensive review on photocatalytic removal of heavy metal ions by polyaniline-based nanocomposites. Chem Eng Commun 1–25. https://doi.org/10.1080/00986445.2023.2227568
Phan Quang HH, Nguyen TP, Duc Nguyen DD et al (2022) Advanced electro-Fenton degradation of a mixture of pharmaceutical and steel industrial wastewater by pallet-activated-carbon using three-dimensional electrode reactor. Chemosphere 297:134074. https://doi.org/10.1016/J.CHEMOSPHERE.2022.134074
Puasa SW, Ismail KN, Mahadi MAA et al (2021) Polynomial regression analysis for the removal of heavy metal mixtures in coagulation/flocculation of electroplating wastewater. Indones J Chem 21:46–56. https://doi.org/10.22146/IJC.52251
Qiao J, Jiang Z, Sun B et al (2012) Arsenate and arsenite removal by FeCl3: effects of pH, As/Fe ratio, initial As concentration and co-existing solutes. Sep Purif Technol 92:106–114. https://doi.org/10.1016/J.SEPPUR.2012.03.023
Ríos G, Pazos C, Coca J (1998) Destabilization of cutting oil emulsions using inorganic salts as coagulants. Colloids Surf A Physicochem Eng Asp 138:383–389. https://doi.org/10.1016/S0927-7757(97)00083-6
Sanin VN, Ikornikov DM, Andreev DE et al (2019) Mill scale recycling by SHS metallurgy for production of cast ferrosilicon and ferrosilicoaluminium. IOP Conf Ser Mater Sci Eng 558:012041. https://doi.org/10.1088/1757-899X/558/1/012041
Schwertmann U, Taylor RM (2018) Iron oxides. Miner Soil Environ 379–438. https://doi.org/10.2136/SSSABOOKSER1.2ED.C8
Semerjian L, Ayoub GM (2003) High-pH–magnesium coagulation–flocculation in wastewater treatment. Adv Environ Res 7:389–403. https://doi.org/10.1016/S1093-0191(02)00009-6
Sen R, Dehiya S, Pandel U, Banerjee MK (2015) Utilization of low grade coal for direct reduction of mill scale to obtain sponge iron: effect of reduction time and particle size. Procedia Earth Planet Sci 11:8–14. https://doi.org/10.1016/J.PROEPS.2015.06.003
Shahid MK, Kim Y, Choi YG (2019) Magnetite synthesis using iron oxide waste and its application for phosphate adsorption with column and batch reactors. Chem Eng Res Des 148:169–179. https://doi.org/10.1016/J.CHERD.2019.06.001
Sirajuddin A, Rathi RK, Umesh C (2010) Wastewater treatment technologies commonly practiced in major steel industries of India. 16th Ann Int Sustain Dev Res Conf 30
SteelData (2023) Türkiye’nin En Büyük Online Çelik İstatistik Merkezi. In: SteelData . https://www.steel-data.com/post/155/2021de-dunya-celik-uretimi-37-artisla. Accessed 10 Mar 2023
Stephenson RJ, Duff SJB (1996) Coagulation and precipitation of a mechanical pulping effluent—I. Removal of carbon, colour and turbidity. Water Res 30:781–792. https://doi.org/10.1016/0043-1354(95)00213-8
Sun W, Xu X, Lv Z et al (2019) Environmental impact assessment of wastewater discharge with multi-pollutants from iron and steel industry. J Environ Manage 245:210–215. https://doi.org/10.1016/J.JENVMAN.2019.05.081
Taheriyoun M, Memaripour A, Nazari-Sharabian M (2020) Using recycled chemical sludge as a coagulant aid in chemical wastewater treatment in Mobarakeh Steel Complex. J Mater Cycles Waste Manag 22:745–756. https://doi.org/10.1007/S10163-019-00966-7/FIGURES/8
Takaya T, Enokida I, Furukawa Y, Iwata K (2019) Direct observation of structure and dynamics of photogenerated charge carriers in poly(3-hexylthiophene) films by femtosecond time-resolved near-IR inverse Raman spectroscopy. Molecules 24:. https://doi.org/10.3390/MOLECULES24030431
Tang W, Li H, Fei L et al (2022) The removal of microplastics from water by coagulation: a comprehensive review. Sci Total Environ 851:158224. https://doi.org/10.1016/J.SCITOTENV.2022.158224
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Heavy metal toxicity and the environment. 133–164
Teh CY, Budiman PM, Shak KPY, Wu TY (2016) Recent advancement of coagulation-flocculation and its application in wastewater treatment. Ind Eng Chem Res 55:4363–4389. https://doi.org/10.1021/ACS.IECR.5B04703/ASSET/IMAGES/MEDIUM/IE-2015-047039_0007.GIF
Tong Y, Cai J, Zhang Q et al (2019) Life cycle water use and wastewater discharge of steel production based on material-energy-water flows: a case study in China. J Clean Prod 241:. https://doi.org/10.1016/J.JCLEPRO.2019.118410
Tran NN, Escribà-Gelonch M, Sarafraz MM et al (2023) Process technology and sustainability assessment of wastewater treatment. Ind Eng Chem Res 62:1195–1214. https://doi.org/10.1021/ACS.IECR.2C03471
Turan MD, Sarı ZA, Nizamoğlu H (2021) Pressure leaching of chalcopyrite with oxalic acid and hydrogen peroxide. J Taiwan Inst Chem Eng 118:112–120. https://doi.org/10.1016/J.JTICE.2020.10.021
Turan MD, Silva JP, Sarı ZA et al (2022) Dissolution of chalcopyrite in presence of chelating agent and hydrogen peroxide. Trans Indian Inst Met 75:273–280. https://doi.org/10.1007/s12666-021-02426-z
Vasilenko TA, Koltun AA (2017) Chemical aspects of the obtaining of iron-containing coagulant-flocculant from electric steel melting slag for wastewater treatment. Solid State Phenom 265:403–409
Verma S, Prasad B, Mishra IM (2010) Pretreatment of petrochemical wastewater by coagulation and flocculation and the sludge characteristics. J Hazard Mater 178:1055–1064. https://doi.org/10.1016/J.JHAZMAT.2010.02.047
Verma AK, Dash RR, Bhunia P (2012) A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J Environ Manage 93:154–168. https://doi.org/10.1016/J.JENVMAN.2011.09.012
Voyiatzis GA, Kalampounias AG, Papatheodorou GN (1999) The structure of molten mixtures of iron(III) chloride with caesium chloride. Phys Chem Chem Phys 1:4797–4803. https://doi.org/10.1039/A905655F
Wei H, Gao B, Ren J et al (2018) Coagulation/flocculation in dewatering of sludge: a review. Water Res 143:608–631. https://doi.org/10.1016/j.watres.2018.07.029
WHO (1996) The world health report: 1996: fighting disease, fostering development. Geneva
WHO (2022) Guidelines for drinking-water quality, fourth. World Health Organization, Geneva
World Steel Association (2022) Steel statistical yearbook. Brussels – Belgium
Xiong Z, Cao J, Yang D et al (2017) Coagulation-flocculation as pre-treatment for micro-scale Fe/Cu/O3 process (CF-mFe/Cu/O3) treatment of the coating wastewater from automobile manufacturing. Chemosphere 166:343–351. https://doi.org/10.1016/J.CHEMOSPHERE.2016.09.038
Xu GR, Yan ZC, Wang YC, Wang N (2009) Recycle of Alum recovered from water treatment sludge in chemically enhanced primary treatment. J Hazard Mater 161:663–669. https://doi.org/10.1016/j.jhazmat.2008.04.008
Zeng Z, Yi L, He J et al (2020) Hierarchically porous carbon with pentagon defects as highly efficient catalyst for oxygen reduction and oxygen evolution reactions. J Mater Sci 55:4780–4791. https://doi.org/10.1007/S10853-019-04327-5/FIGURES/5
Zhang J, Chen G, Ma Y et al (2022b) Purification of pickling wastewater from the steel industry using membrane filters: performance and membrane fouling. Environ Eng Res 27:200486. https://doi.org/10.4491/EER.2020.486
Zhang B, Liu B, Huang Y et al (2022a) Recovery of molybdenum from metallurgical wastewater by Fe(III) coagulation and precipitation flotation process. Miner Met Mater Ser 251–258. https://doi.org/10.1007/978-3-030-92563-5_26/COVER
Zhao C, Zhou J, Yan Y et al (2021) Application of coagulation/flocculation in oily wastewater treatment: a review. Sci Total Environ 765:142795. https://doi.org/10.1016/J.SCITOTENV.2020.142795
Acknowledgements
The authors would like to thank Iskenderun Technical University for their support.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
Alper Solmaz: conceptualization, original draft, formal analysis, data curation, writing—review and editing.
Ömer Saltuk Bölükbaşı: data curation, result interpretation, writing—review and editing.
Zeynel Abidin Sarı: Conceptualization, original draft, formal analysis, investigation, data curation, writing—review and editing.
Corresponding author
Ethics declarations
All authors have read, understood, and have complied as applicable with the statement on “Ethical responsibilities of Authors” as found in the Instructions for Authors and are aware that with minor exceptions, no changes can be made to authorship once the paper is submitted.
Ethical approval
Not applicable.
Consent to participate
All authors have given consent to their contribution.
Consent for publication
All authors have agreed with the content and given explicit consent to publish.
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Guilherme Luiz Dotto
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Solmaz, A., Bölükbaşi, Ö.S. & Sari, Z.A. Green industry work: production of FeCl3 from iron and steel industry waste (mill scale) and its use in wastewater treatment. Environ Sci Pollut Res 31, 19795–19814 (2024). https://doi.org/10.1007/s11356-024-32451-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-024-32451-6