Abstract
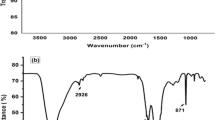
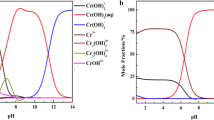
The present paper describes the development of a new chemically modified carbon paste electrode based on azithromycin (AMC) that considered as a selective aluminium recognition agent in the carbon paste electrode (CPE). This electrode was fully characterized in terms of composition, response time, usable pH range and temperature. The electrode exhibited a Nernstian response for Al3+ ion over a concentration range from 7.0 × 10–6 to 1.0 × 10–2 mol L–1 with a slope of 21.3 ± 0.18 mV decade–1 and the limit of detection was 6.0 × 10–6 mol L−1. It had a response time of about 6 s. The proposed sensor possesses a very good selectivity with respect to a variety of other cations. Finally this modified electrode was applied for the determination of the concentration of Al3+ ion in different water samples and the obtained results were comparable with those obtained with atomic absorption spectrometer (AAS).
Similar content being viewed by others
References
Guidelines for Drinking-Water Quality, Health Criteria and Other Supporting Information, Addendum, 1998, Sec. Edition, vol.2.
Soleimani, M. and Afshar, M.G., Octaethylporphyrin as an ionophore for aluminium potentiometric sensor based on carbon paste electrode, Russian in Elektrokhimiya, 2014, vol. 50, pp. 554–560.
Bamji, N.M.S. and Kaladhar, M., Risk of increased aluminium burden in the Indian population: contribution from aluminium cookware, Food Chem., 2000, vol. 70, pp. 57–61.
Arvand, M. and Asadollahzade, S.A., Ion-selective electrode for aluminium determination in pharmaceutical substances, tea leaves and water samples, Talanta, 2008, vol. 75, pp. 1046–1054.
López, F. F., Cabrera, C., Lorenzo, M.L., and López, M.C., Aluminium content of drinking waters, fruit juices and soft drinks: contribution to dietary intake, Sci. Total Environ., 2002, vol. 292, pp. 205–213.
Jalbani, N., Kazi, T.G., Jamali, M.K., Arainm, B.M., Afridi, H.I., and Baloch, A., Evaluation of aluminium contents in different bakery foods by electrothermal atomic absorption spectrometer, J. Food Comp. and Anal., 2007, vol. 20, pp. 226–231.
Malik, J., Frankova, A., Drabek, O., Szakova, J., Ash, C., and Kokoska, L., Aluminium and other elements in selected herbal tea plant species and their infusions, Food Chem., 2013, vol. 139, pp. 728–734.
Scancar, J., Stibilj, V., and Milacic, R., Determination of aluminium in Slovenian foodstuffs and its leachability from aluminium cookware, Food Chem., 2004, vol. 85, pp. 151–157.
Heena Kumar, R., Rani, S., and Malik, A., Development of a rapid and sensitive method for the determination of aluminium by reverse-phase high-performance liquid chromatography using a fluorescence detector, J. Chromatogr. Sci., 2014.
Beltagi, A.M. and Ghoneim, M.M., Simultaneous determination of trace aluminium (III), copper (II) and cadmium (II) in water samples by square-wave adsorptive cathodic stripping voltammetry in the presence of oxine, J. Appl. Electrochem., 2009, vol. 39, pp. 627–636.
Mersal, G.A.M. and Arida, H.A., New carbon paste modified micro electrode based on haematoxylin for determination of aluminium in underground water, Int. J. Electrochem. Sci., 2011, vol. 6, pp. 1116–1126.
Liu, J., Bi, S., Yang, L., Gu, X., Ma, P., Gan, N., Wang, X., Longa, X., and Zhanga, F., Speciation analysis of aluminium(III) in natural waters and biological fluids by complexing with various catechols followed by differential pulse voltammetry detection, Anal., 2002, vol. 12, pp. 1657–1665.
Tajik, S., Taher, M.A., and Sheikhshoaie, I., Potentiometric determination of trace amounts of aluminium utilizing polyvinyl chloride membrane and coated platinum sensors based on E-N'-(2-hydroxy-3-methoxybenzylidene) benzohydrazide, J. AOAC Int., 2013, vol. 96, pp. 1204–1211.
Bera, R.K., Sahoo, S.K., Mittal, S.K., and Kumar, A.S.K., An imidazol based novel potentiometric PVC membrane sensor for aluminium(III) determination, Int. J. Electrochem. Sci., 2010, vol. 5, pp. 29–38.
Aglan, R.F., Mohamed, G.G., and Mohamed, H.A., Chemically modified carbon paste electrode for determination of cesium ion by potentiometric method, American Journal of Analytical Chemistry, 2012, vol. 3, pp. 576–586.
Arvand, M. and Kermanian, M., Potentiometric determination of aluminum in foods, pharmaceuticals, and alloys by AlMCM-41-modified carbon paste electrode, Food Analytical Methods, 2012, vol. 6, pp. 578–586.
Li, Y., Chai, Y., Yuan, R., Liang, W., Zhang, L., and Ye, G., Aluminium(III)-selective electrode based on a newly synthesized glyoxal-bis-thiosemicarbazone Schiff base, J. A. CHEM, 2008, vol. 63, p. 1090.
Evsevleeva, L., Bykova, L., and Badenikov, V., Aluminum-selective electrode, J. A. CHEM, 2005, vol. 60, no. 9, p. 866.
Bratovcic, A., Odobaš ic, A., and Catic, S., The advantages of the use of ion-selective potentiometry in relation to UV/Vis spectroscopy, Agriculturae Conspectus Scientificus, 2009, vol. 3, pp. 139–142.
Nour El Dein, F.A., Mohamed, G.G., Frag, E.Y.Z., and Mohamed, M.E., Modified screen printed and carbon paste ion selective electrodes for potentiometric determination of naphazoline hydrochloride in pure and pharmaceutical preparations, Int. J. Electrochem. Sci., 2012, vol. 7, pp. 10266–10281.
Umezawa, Y., Umezawa, K., and Sato, H., Selectivity coefficients for ion selective electrodes: Recommended methods for reporting kpot values, Pure and Applied Chemistry, 1995, vol. 67, pp. 507–518.
Arayne, S.M., Sultana, N., Shamim, S., and Naz, A., Synthesis characterization and antimicrobial activities of azithromycin metal complexes, Modern Chem. & Appl., 2014, p. 3.
Mihali, C. and Vaum, N., Use of plasticizer for electrochemical sensors, Recent Advances in Plasticizers, Dr. Mohammad Luqman, Ed., 2012, ISBN 978-953-51-0363-9.
Antropov, L.I., Theo. Electrochem., Moscow: Mir, 1972.
Bard, A.J., Parson, R., and Jordan, J., Standard Potentials in Aqueous Solution, Marcel Dekker, Inc., 1985.
VIM, International vocabulary of metrology—Basic and general concepts and associated terms, JCGM, 3rd ed., 2012.
Gumustas, M. and Ozkan, S.A., The role of and the place of method validation in drug analysis using electroanalytical techniques, Open Anal. Chem. J., 2011, vol. 5, pp. 1–21.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Elektrokhimiya, 2016, Vol. 52, No. 8, pp. 843–850.
The article is published in the original.
Rights and permissions
About this article
Cite this article
Mohamed, M.E. Modified carbon paste electrode for potentiometric determination of aluminium ion in spiked real water sample. Russ J Electrochem 52, 754–761 (2016). https://doi.org/10.1134/S1023193516080073
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1023193516080073