Abstract
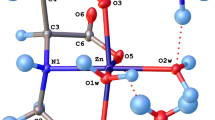
The complex between carbonic anhydrase enzyme center (CA) and a derivative of fullerene as a nanoscale inhibitor (C60-Inh) has been investigated, based on, B3LYP level, using 6-31G* basis set. The results of calculations indicate that this special fullerene derivative could be deprotonated from three different positions and interacts with CA active site to form three CA-C60-Inh complexes. The calculated results indicate that deprotonated inhibitor is coordinated to the Zn2+ ion and all the complexes have tetrahedral geometry. The calculated binding energy (BD) and complexation energy clearly show the complex between C60-Inh and CA active site from N13 position is more favorable than the other position. Also thermodynamic functions such as standard enthalpy of complexation (ΔH 0com ), standard entropy of complexation (ΔS 0com ) and standard Gibbs free energy of complexation (ΔG 0com ) for three CA-inhibitor complexes are evaluated. In order to approach the ideal geometry and provide further insight into the different complexation properties, the single point calculation at the B3LYP/6-311G** level have been used for all three different complexes to confirm the results of B3LYP/6-31G*. Thus, fullerene derivatives show a new class of nano scale carbonic anhydrase inhibitors that might find applications for targeting physiologically relevant isoforms of different forms of CA.
Similar content being viewed by others
References
S. Parkkila, A. K. Parkkila, and J. Kivela, in: Carbonic Anhydrase: Its Inhibitors and Activators, Eds. C. T. Supuran, A. Scozzafava and J. Conway, CRC Press: Boca Raton (2004), p. 283–301
K. Kaila and B. Ransom, PH and Brain Function, Wiley-Liss, New-York (1998).
P. Halmi, S. Parkkila, and J. Honkaniemi, Neurochem. Int., 48, 24–30 (2006).
J. F. Domsic, B. S. Avvaru, C. U. Kim, et al., J. Biol. Chem., 283, 30766–30771 (2008).
J. F. Ferry, Biochim. Biophys. Acta, 1804, 374–381 (2010)
K. S. Smith, C. Jakubzick, T. S. Whittam, et al., Proc. Natl. Acad. Sci., U.S.A. (1999), 96, 5184–15189
S. A. Zimmerman, J. F. Tomb, and J. G. Ferry, J. Bacteriol., 192, 1353–1360 (2010)
S. A. Zimmerman, J. G. Ferry, and C. T. Supuran, Curr. Top. Med. Chem., 7, 901–908 (2007)
S. Elleuche and S. Pöggeler, Microbiology., 156, 23–29 (2010).
S. Lindskog, Pharmacol. Ther., 74, 1–20 (1997).
D. N. Silverman and S. Lindskog, Acc. Chem. Res., 21, 30–36 (1988).
A. Liljas, K. K. Kannan, P. C. Bergsten, et al., Nature, 235, 131–137 (1972).
S. K. Nair and D. W. Christianson, J. Am. Chem. Soc., 113, 9455–9458 (1991).
Z. Fisher, J. A. Hernandez Prada, C. Tu, et al., Biochemistry, 44, 1097–1105 (2005).
H. Steiner, B. H. Jonsson, and S. J. Lindskog, Eur. J. Biochem., 59, 253–259 (1975).
C. Tu, D. N. Silverman, C. Forsman, et al., Biochemistry, 28, 7913–7918 (1989).
D. Duda, C. Tu, M. Qian, et al., Biochemistry, 40, 1741–1748 (2001).
C. T. Supuran and A. Scozzafava, Bioorg. Med. Chem., 15, 4336–4350 (2007).
S. Pastorekova, S. Parkkila, J. Pastorek, et al., J. Enzyme Inhib. Med. Chem., 19, 199–229 (2004).
C. T. Supuran, Nat. Rev. Drug Disc., 7, 168–181 (2008).
C. T. Supuran, Carbonic Anhydrases as Drug Targets-General Presentation. In: Drug Design of Zinc-Enzyme Inhibitors: Functional, Structural, and Disease Applications (C. T. Supuran., J. Y. Winum Eds.), Wiley: Hoboken (NJ) (2009), p. 15–38
J. Y. Winum, M. Rami, A. Scozzafava, et al., Med. Res. Rev., 28, 445–463 (2008)
C. T. Supuran, A. Scozzafava, and A. Casini, Med. Res. Rev., 23, 146–189 (2003)
J. F. Domsic, B. S. Avvaru, C. U. Kim, et al., J. Biol. Chem., 283, 30766–30771 (2008).
C. T. Supuran, Curr. Pharm. Des., 14, 641–648 (2008)
C. T. Supuran, A. Di Fiore, and G. De Simone, Expert Opin. Emerg. Drugs., 13, 383–392 (2008)
G. De Simone, A. Di Fiore, and C. T. Supuran, Curr. Pharm. Des., 14, 655–660 (2008)
F. Mincione, A. Scozzafava, and C. T. Supuran, Antiglaucoma Carbonic Anhydrase Inhibitors as Ophthalmologic Drugs. In: Drug Design of Zinc-Enzyme Inhibitors: Functional, Structural, and Disease Applications (C. T. Supuran and J. Y. Winum Eds.), Wiley: Hoboken (NJ) (2009), p. 139–154
J. Krungkrai and C. T. Supuran, Curr. Pharm. Des., 14, 631–640 (2008)
J. Borras, A. Scozzafava, L. Menabuoni, et al., Bioorg. Med. Chem., 7, 2397–2406 (1999).
I. Nishimori, S. Onishi, H. Takeuchi, et al., Curr. Pharm. Des., 14, 622–630 (2008)
C. Schlicker, R. A. Hall, D. Vullo, et al., J. Mol. Biol., 385, 1207–1220 (2009)
S. Isik, F. Kockar, M. Aydin, et al., Bioorg. Med. Chem., 17, 1158–1163 (2009)
F. Carta, A. Maresca, A. Suarez Covarrubias, et al., Bioorg. Med. Chem. Lett., 19, 6649–6654 (2009).
B. W. Clare and C. T. Supuran, J. Pharm. Sci., 83, 768–773 (1994)
C. Temperini, D. Vullo, A. Scozzafava, et al., J. Med. Chem., 49, 3019–3027 (2006).
A. V. Luzanov, J. Struct. Chem., 43, 1–9 (2002).
V. N. Ivanova, J. Struct. Chem., 41, 135–148 (2000).
Y. Tabata and Y. Ikada, Pure Appl. Chem., 71, 2047–2053 (1999).
S. H. Friedman, D. L. De Camp, R. P. Sijbesma, et al., J. Am. Chem. Soc., 115, 6506–6509 (1993).
H. Tokuyama, S. Yamago, E. Nakamura, et al., J. Am. Chem. Soc., 5, 7918/7919 (1993).
I. Wang, I. L. Tai, D. Lee, et al., J. Med. Chem., 42, 4614–4620 (1999).
S. T. Yang, H. F. Wang, L. Guo, et al., Nanotechnology, 19, 395101–395108 (2008).
A. Innocenti, S. Durdagi, N. Doostdar, et al., Bioorg. Med. Chem., 18, 2822–2828 (2010).
S. R. Wilson, D. I. Schuster, B. Nuber, et al., Fullerenes: Chemistry, Physics, and Technology (K. Kadish and R. Ruoff Eds.), John Wiley & Sons, New-York (2000).
W. Kratschmer, L. D. Lamb, and D. R. Hoffman, Nature, 347, 354–358 (1990).
S. Yamago, H. Tokuyama, E. Nakamura, et al., Chem. Biol., 2, 385–389 (1995).
C. T. Supuran, Bioorg. Med. Chem. Lett., 20, 3467–3474 (2010).
S. Bosi, T. Da Ros, G. Spalluto, et al., Eur. J. Med. Chem., 38, 913–923 (2003)
D. Pantarotto, N. Tagmatarchis, A. Bianco, et al., Mini-Rev. Med. Chem., 4, 805–814 (2004)
L. Sanchez, R. Otero, J. M. Gallego, et al., Chem. Rev., 109, 2081–2091 (2009).
B. Kang, D. Yu, Y. Dai, et al., Small., 5, 1292–1301 (2009)
P. Chaudhuri, A. Paraskar, S. Soni, et al., ACS. Nano., 3, 2505–2014 (2009)
P. Mroz, G. P. Tegos, H. Gali, et al., Photochem. Photobiol. Sci., 6, 1139–1149 (2007).
K. Yudoh, R. Karasawa, K. Masuko, et al., Int. J. Nanomed., 4, 217–225 (2009)
R. Partha and J. L. Conyers, Int. J. Nanomed., 4, 261–275 (2009)
K. Yudoh, K. Shishido, H. Murayama, et al., Arthritis Rheum., 56, 3307–3318 (2007).
J. Yang and A. R. Barron, Chem. Commun., 21, 2884/2885 (2004)
J. Yang, L. B. Alemany, and J. Driver, Chem. Eur. J., 13, 2530–2545 (2007)
J. Yang, K. Wang, J. Driver, et al., Org. Biomol. Chem., 5, 260–266 (2007).
M. Ghiasi, S. Kamalinahad, M. Arabieh, and M. Zahedi, Comput. Theo. Chem., 992, 59–69 (2012).
M. J. Frisch, G. W. Trucks, H. B. Schlegel, et al., Gaussian 2003 (Revision-B), Gaussian, Inc., Pittsburgh PA (2003).
A. D. Beck, J. Chem. Phys., 98, 5648–5652 (1993).
R. G. Parr and W. Yang, Density-functional theory of atoms and molecules, Oxford Univ. Press (1989).
D. J. Barbiric, E. A. Castro, and R. H. de Rossi, J. Mol. Struct: Theochem., 532, 171–181 (2000).
M. Navarrete, C. J. C. Rangel, and J. Corchado, J. Phys. Chem. A, 109, 4777–4784 (2005).
A. K. Chandra and T. Uchimaru, Int. J. Mol. Sci., 3, 407–422 (2002).
H. Y. Zhang and H. F. Ji, J. Mol. Struct: Theochem., 663, 167–174 (2003).
M. Ghiasi, M. Taheri, and M. Zahedi, Comp. Theo. Chem., 1022, 121–129 (2013).
A. Casini, J. Antel, F. Abbate, et al., Bioorg. Med. Chem. Lett., 13, 841–845 (2003).
M. Bialer, S. I. Johannessen, H. J. Kupferberg, R. H. Levey, P. Loiseau, and E. Perucca, Epilepsy Res., 43, 11–58 (2001).
J. L. Stringer, Epilepsy Res., 40, 147–153 (2000).
A. Sabers and L. Gram, Drugs., 60, 23–33 (2000).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © 2014 M. Ghiasi, S. Kamalinahad, M. Zahedi.
The text was submitted by the authors in English. Zhurnal Strukturnoi Khimii, Vol. 55, Supplement 2, pp. S392–S403, 2014.
Rights and permissions
About this article
Cite this article
Ghiasi, M., Kamalinahad, S. & Zahedi, M. Complexation of nanoscale enzyme inhibitor with carbonic anhydrase active center: A quantum mechanical approach. J Struct Chem 55, 1574–1586 (2014). https://doi.org/10.1134/S0022476614080277
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022476614080277