Abstract
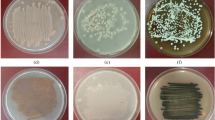
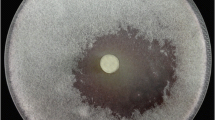
Actinomycetes were screened from soil in the centre of Poland on chitin medium. Amongst 30 isolated strains one with high activity of chitinase was selected. It was identified as Streptomyces sporovirgulis. Chitinase activity was detected from the second day of cultivation, then increased gradually and reached maximum after 4 days. The maximum chitinase production was observed at pH 8.0 and 25–30°C in the medium with sodium caseinate and asparagine as carbon and nitrogen sources and with shrimp shell waste as inducer of enzyme. Chitinase of S. sporovirgulis was purified from a culture medium by fractionation with ammonium sulphate as well as by chitin affinity chromatography. The molecular weight of the enzyme was 27 kDa. The optimum temperature and pH for the chitinase were 40°C and pH 8.0. The enzyme activity was characterised by high stability at the temperatures between 35 and 40°C after 240 min of preincubation. The activity of the enzyme was strongly inhibited in the presence of Pb2+, Hg2+ and stabilized by the ions Mg2+. Purified chitinase from S. sporovirgulis inhibited growth of fungal phytopathogen Alternaria alternata. Additionally, the crude chitinase inhibited the growth of potential phytopathogens such as Penicillium purpurogenum and Penillium sp.
Similar content being viewed by others
References
Gohel, V., Singh, A., Vimal, M., Ashwini, P., and Chhatpar, H.S., Afr. J. Biotechnol., 2006, vol. 5, no. 2, pp. 54–72.
Patil, R.S., Ghormade, V., and Deshpande, M.V., Enzyme Microb. Tech., 2000, vol. 26, no. 7, pp. 473–483.
Dahiya, N., Tewari, R., and Hoondal, G.S., Appl. Microbiol. Biotechnol., 2006, vol. 71, no. 6, pp. 773–782.
Dahiya, N., Tewari, R., Tiwari, R.P., and Hoondal, G.S., World J. Microbiol. Biotechnol., 2005, vol. 21, nos. 8–9, pp. 1611–1616.
Strange, R.N. and Scott, P.R., Annu. Rev. Phytopathol., 2005, vol. 43, pp. 83–116.
Budi, S.W., van Tuinen, D., Arnould, C., Dumas-Gaudut, E., Gianinazzi-Pearson, V., and Gianinazzi, S., Appl. Soil Ecol., 2000, vol. 15, no. 2, pp. 191–199.
Lingappa, Y. and Lockwood, J.L., Phytopatology, 1962, vol. 52, pp. 317–323.
Williams, S.T., Goodfellow, M., Alderson, G., Wellington, E.M.H., Sneath, P.H.A., and Sackin, M., J. Gen. Microbiol., 1983, vol. 129, no. 6, pp. 1743–1813.
Watanabe, N., Kodama, Y., and Harayama, S., J. Microbiol. Methods, 2001, vol. 44, no. 3, pp. 253–262.
Kutchma, A.J., Roberts, M.A., Knaebel, D.B., and Crawford, D.L., Biotechniques, 1998, vol. 24, no. 3, pp. 452–456.
Escott, G.M., Hearn, V.M. and Adams, D.J., Microbiology, 1998, vol. 144, no. 6, pp. 1575–1581.
Bradford, M.M., Anal. Biochem., 1976, vol. 72, nos. 1–2, pp. 248–254.
Laemmli, U.K., Nature, 1970, vol. 227, pp. 680–688.
Roberts, W.K. and Selitrennikoff, C.P., J. Gen. Microbiol., 1988, vol. 134, no. 1, pp. 169–176.
Barnett, H.L. and Barry, B.H., Illustrated Genera of Imperfect Fungi, 4th ed., St. Paul: The American Phytopathological Society, 1998.
Fassatiova, O., Microscopic Fungi in Technical Microbiology, Warsaw: Scientific Publications Technical., 1983.
Kwas’na, H., Che kowski, J., and Zajkowski, P., Fungi (Mycota), Warsaw-Krakow: Polish Scientific Publ., 1991, vol. 22.
Piontek, M., Mould Fungi, Zielona Góra: Publisher’s Atlas by the University of Zielona Góra, 1999.
Mane, U.V. and Deshmukh, A.M., Afr. J. Biotechnol., 2009, vol. 8, no. 23, pp. 6617–6620.
Margino, S., Nugroho, A.J., and Asmara, W., Ind. J. Biotechnol., 2010, vol. 15, no. 1, pp. 29–36.
Shanmugaiah, V., Mathivanan, N., Balasubramanian, N., and Manoharan, P.T., Afr. J. Biotechnol., vol. 7, no. 15, pp. 2562–2568.
Hosny, M.S., El-Shayeb, N.A., Abood, A., and Abdel-Fattah, A.M., Aust. J. Basic Appl. Sci., 2010, vol. 4, no. 4, pp. 615–623
Chang, W.T., Chen, Y.C., and Jao, C.L., Biores. Technol., 2007, vol. 98, no. 6, pp. 1224–1230.
Rattanakit, N., Plikomol, A., Yano, S., Wakayama, M., and Tachiki, T., J. Biosc. Bioeng., 2002, vol. 93, no. 6, pp. 550–556.
Mukherjee, G. and Sen, S.K., Curr. Microbiol., 2006, vol. 53, no. 4, pp. 265–269.
Nawani, N.N., and Kapadnis, B.P., J. Appl. Microbiol., 2001, vol. 90, no. 5, pp. 803–808.
Aly, M.M., Tork, S., Al-Garni, S.M., and Kabli, S.A., Ann. Microbiol., 2011, vol. 61, no. 3, pp. 453–461.
Jiang, X., Chen, D., Hong, S., Wang, W., Chen, S., and Zou, S., Carbohydr. Polym., 2012, vol. 87, no. 4, pp. 2409–2415.
Bhattacharya, D., Nagpure, A., and Gupta, R.K., Crit. Rev. Biotechnol., 2007, vol. 27, no. 1, pp. 21–28.
Rabeeth, M., Anitha, A., and Srikanth, G., Pakistan J. Biol. Sci., 2011, vol. 14, no. 16, pp.788–797.
Kim, K.J, Yang, Y.J, and Kim, J.G., J. Biochem. Mol. Biol., 2003, vol. 36, no. 2, pp. 185–189.
Bhushan, B. and Hoondal, G.S., Biotechnol. Lett., 1998, vol. 20, no. 2, pp. 157–159.
Vaidya, R.J., Shah, I.M., Vyas, P.R., and Chhatpar, H.S., World J. Microbiol. Biotechnol., 2001, vol. 17, no. 7, pp. 691–696.
Baharlouei, A., Sharifi-Sirchi, G.R., and Shahidi Bonjar, G.H., Philipp. Agric. Scientist., 2011, vol. 93, no. 4, pp. 439–445.
Ghasemi, S., Ahmadian, G., Jelodar, N.B., Rahimian, H., Ghandili, S., Dehestani, A., and Shariati, P., World J. Microbiol. Biotechnol., 2010, vol. 26, no. 8, pp. 1437–1443.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Swiontek Brzezinska, M., Jankiewicz, U. & Lisiecki, K. Optimization of cultural conditions for the production of antifungal chitinase by Streptomyces sporovirgulis . Appl Biochem Microbiol 49, 154–159 (2013). https://doi.org/10.1134/S0003683813020014
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683813020014