Abstract
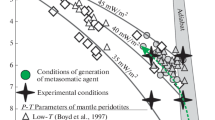
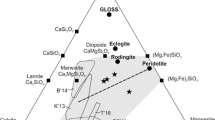
Generation of ultra-alkaline melts by the interaction of lherzolite with cardonatites of various genesis was simulated at the P–T parameters typical of the base of the subcratonic lithosphere. Experiments with a duration of 150 h were performed at 5.5 and 6.3 GPa and 1350°C. The concentrations of CaO and MgO in melts are buffered by the phases of peridotite, and the concentrations of alkalis and FeO depend on the composition of the starting carbonatite. Melts are characterized by a low (<7 wt %) concentration of SiO2 and Ca# from 0.40 to 0.47. It is demonstrated that only high-Mg groups of carbonatitic inclusions in fibrous diamonds have a composition close to that of carbonatitic melts in equilibrium with lherzolite. Most likely, the formation of kimberlite-like melts relatively enriched in SiO2 requires an additional source of heat from mantle plumes and probably H2O fluid.
Similar content being viewed by others
References
M. E. Wallace and D. H. Green, Nature 335, 343–346 (1989).
M. Becker and A. P. Le Roex, J. Petrol. 47, 673–703 (2006).
L. S. Doucet, D. A. Ionov, and A. V. Golovin, Contrib. Mineral. Petrol. 165, 1225–1242 (2013).
G. P. Brey, V. K. Bulatov, and A. V. Girnis, Lithos 112, 249–259 (2009).
A. M. Logvinova, R. Wirth, E. N. Fedorova, and N. V. Sobolev, Eur. J. Mineral. 20, 317–331 (2008).
M. Kopylova, M. Navon, L. Dubrovinsky, and G. Khachatryan, Earth Planet. Sci. Lett. 291, 126–137 (2010).
G. P. Brey, V. K. Bulatov, and A. V. Girnis, Chem. Geol. 281, 333–342 (2011).
S. Foley, Lithos 28, 435–453 (1992).
D. Grassi and M. W. Schmidt, J. Petrol. 52, 765–789 (2011).
S. R. Hart and A. Zindler, Chem. Geol. 57, 247–267 (1986).
Y. N. Palyanov, Y. M. Borzdov, A. F. Khokhryakov, I. N. Kupriyanov, and A. G. Sokol, Cryst. Growth Des. 10, 3169–3175 (2010).
J. Hernlund, K. Leinenweber, D. Locke, and J. A. Tyburczy, Am. Mineral. 91, 295–305 (2006).
N. V. Sobolev, Yu. G. Lavrentiev, L. N. Pospelova, and E. V. Sobolev, Dokl. Akad. Nauk SSSR 189 1, 162–165 (1969).
R. Dasgupta and M. M. Hirschmann, Contrib. Mineral. Petrol. 154, 647–661 (2007).
A. G. Sokol, A. N. Kruk, D. A. Chebotarev, Yu. N. Palyanov, and N. V. Sobolev, Dokl. Earth Sci. 465 2, 1262–1267 (2015).
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.N. Kruk, A.G. Sokol, D.A. Chebotarev, Yu.A. Palyanov, N.V. Sobolev, 2016, published in Doklady Akademii Nauk, 2016, Vol. 467, No. 3, pp. 324–328.
Rights and permissions
About this article
Cite this article
Kruk, A.N., Sokol, A.G., Chebotarev, D.A. et al. Composition of a carbonatitic melt in equilibrium with lherzolite at 5.5–6.3 GPa and 1350°C. Dokl. Earth Sc. 467, 303–307 (2016). https://doi.org/10.1134/S1028334X16030181
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1028334X16030181