Abstract
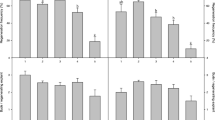
In vitro regeneration techniques have been optimized for seven strains and cultivars of sugar beet (Beta vulgaris L.) bred in Russia. The frequency of shoot regeneration from somatic cells and tissues of sugar beet varies from 10 to 97% depending on the explant type, culture-medium composition, and genotype. The in vitro regeneration potential has been estimated in plants with different genotypes. The effect of medium composition (phytohormones and carbohydrates) on the frequency of the formation of a morphogenic callus competent for plant regeneration has been determined. The effect of the types and concentrations of various cytokines (zeatin, kinetin, and 6-benzylaminopurine) on direct shoot regeneration from cotyledon nodes has been estimated. The culture-medium composition has been optimized for direct shoot regeneration from petioles. The effects of different concentrations of abscisic acid on the frequency of shoot regeneration from a morphogenic callus has been studied. Micropropagation has been used to obtain petiole explants and reproduce the shoots obtained by direct regeneration from cotyledonnodes, petioles, and calluses. Improved shoot-regeneration methods can be used for both agrobacterial and bioballistic genetic transformation of the sugar beet genotypes studied.
Similar content being viewed by others
References
Butenko, R.G., Atanassov, A.I., and Urmantseva, V.V., Some Feature of Sugarbeet Tissue Cultures, Phytomorphology, 1972, vol. 22, pp. 140–143.
Welander, T., Callus and Root Formation in Explants of Beta vulgaris L., Physiol. Plant., 1974, vol. 32, pp. 305–307.
Hooker, M.P. and Nabors, M.W., Callus Initiation, Growth, and Organogenesis in Sugar Beet (Beta vulgaris L.), Z. Pflanzenphysiol., 1977, vol. 84, pp. 237–246.
Catlin, D.W., The Effect of Antibiotics on the Inhibition of Callus Induction and Plant Regeneration from Cotyledons of Sugar Beet (Beta vulgaris L.), Plant Cell Rep., 1990, vol. 9, pp. 285–288.
Jacq, B., Tetu, T., Sangwan, R.S., et al., Plant Regeneration from Sugar Beet (Beta vulgaris L.) Hypocotyls Cultured in Vitro and Flow Cytometric Nuclear DNA Analysis of Regenerants, Plant Cell Rep., 1992, vol. 11, pp. 329–333.
Snyder, G.W., Ingersoll, J.C., Smigocki, A.C., and Owens, L.D., Introduction of Pathogen Defense Genes and a Cytokinin Biosynthesis Gene into Sugar Beet (Beta vulgaris L.) by Agrobacterium or Particle Bombardment, Plant Cell Rep., 1999, vol. 18, pp. 829–834.
Freytag, A.H., Anan, S.C., Rao-Ardelli, A.P., and Owens, L.D., An Improved Medium for Adventitious Shoot Formation and Callus Induction in Beta vulgaris L.: In Vitro, Plant Cell Rep., 1988, vol. 7, pp. 30–34.
Bannikova, M.A., Golovko, A.E., Khvedynich, O.A., et al., In Vitro Organogenesis and Protoplast Cultivation of Sugar Beet (Beta vulgaris L.), Abstracts of IV Eur. Cell Biol. Congr., Prague, 1994, p. 546.
Krens, F.A. and Jamar, D., The Role of Explant Source and Culture Conditions on Callus Induction and Shoot Regeneration in Sugar Beet (Beta vulgaris L.), J. Plant Physiol., 1989, vol. 134, pp. 651–655.
Fry, J.E., Barnason, A.R., and Hinchee, M., Genotype-Independent Transformation Using Agrobacterium tumefaciens, Molecular Biology of Plant Growth and Development: 3rd Int. Congr. Int. Society for Plant Mol. Biol., Hallick, R.B., Ed., Tucson, United States, 1991, abstract no. 384.
Krens, F.A., Trifonova, A., Keizer, L.C.P., and Hall, R.D., The Effect of Exogenously-Applied Phytohormones on Gene Transfer Efficiency in Sugar Beet (Beta vulgaris L.), Plant Sci., 1996, vol. 116, pp. 97–106.
Toldi, O., Gyulai, G., Kiss, J., et al., Antiauxin-Enhanced Microshoot Initiation and Plant Regeneration from Epicotyl-Originated Thin-Layer Explants of Sugar Beet (Beta vulgaris L.), Plant Cell Rep., 1996, vol. 15, pp. 851–854.
Murashige, T. and Skoog, F., A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Cultures, Plant Physiol., 1962, vol. 15, pp. 473–476.
Gamborg, O., The Effects of Amino Acid and Ammonium on the Growth of Plant Cells in Suspension Cultures, Plant Physiol., 1970, vol. 45, pp. 372–375.
Arnold, S. and Hakman, I., Regulation of Somatic Embryo Development in Picea abies by Abscisic Acid (ABA), J. Plant Physiol., 1988, vol. 132, pp. 164–169.
Reidiboym-Talleux, L., Diemer, F., Sourdioux, M., et al., Improvement of Somatic Embryogenesis in Wild Cherry (Prunus avium): Effect of Maltose and ABA Supplements, Plant Cell, Tissue Organ Cult., 1999, vol. 55, pp. 199–209.
Langhansova, L., Konradova, H., and Vanek, T., Polyethylene Glycol and Abscisic Acid Improve Maturation and Regeneration of Panax ginseng Somatic Embryos, Plant Cell Rep., 2004, vol. 22, pp. 725–730.
Linossier, L., Veisseire, P., Cailloux, F., and Coudret, A., Effects of Abscisic Acid and High Concentrations of PEG on Hevea brasiliensis Somatic Embryos Development, Plant Sci., 1997, vol. 124, pp. 183–191.
Saunders, J.W. and Daub, M.E., Shoot Regeneration from Hormone-Autonomous Callus from Shoot Cultures of Several Sugar Beet (Beta vulgaris L.) Genotypes, Plant Sci. Lett., 1984, vol. 34, pp. 219–223.
Detrez, C., Tetu, T., Sangwan, R.S., and Sangwan-Norrel, B.S., Direct Organogenesis from Petiole and Thin Cell Layer Explants in Sugar Beet Cultured in Vitro, J. Exp. Bot., 1988, vol. 39, no. 204, pp. 917–926.
Author information
Authors and Affiliations
Additional information
Original Russian Text © Ya.V. Mishutkina, A.K. Gaponenko, 2006, published in Genetika, 2006, Vol. 42, No. 2, pp. 210–218.
Rights and permissions
About this article
Cite this article
Mishutkina, Y.V., Gaponenko, A.K. Sugar beet (Beta vulgaris L.) morphogenesis in vitro: Effects of phytohormone type and concentration in the culture medium, type of explants, and plant genotype on shoot regeneration frequency. Russ J Genet 42, 150–157 (2006). https://doi.org/10.1134/S1022795406020086
Received:
Issue Date:
DOI: https://doi.org/10.1134/S1022795406020086