Abstract
This short review provides an introduction to the rapidly developing field of generation and utilization of “camel nanoantibodies” (or “nanobodies”). The term “nanoantibody” or “nanobody” was given to single-domain variable fragments of special type of antibodies that naturally exist (in addition to classical types of antibodies) in blood of Camelidae family animals and in some chondrichthyan fishes. The existence of very efficient technology of nanobody generation and some very useful characteristic features promise a big potential for their use in immunobiotechnology and medicine.
Similar content being viewed by others
Abbreviations
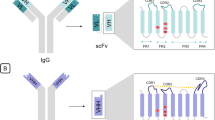
- VH and VL:
-
variable (V) domain of the heavy (H) and of the light (L) chains of the conventional antibody (immunoglobulin)
- CH and CL:
-
constant (C) domains of the heavy and of the light chains, correspondingly
- HCAb:
-
Heavy-chain antibody consisted of a dimer of only shortened (without CH1 domain) heavy chain
- VHH:
-
variable domain of the heavy chain of the Heavy-chain antibody (VHH is the antigen binding region, it has also other names: “nanobody”, “single domain antibody”, “nanoantibody”)
- Fab (Fragment antigen binding):
-
antigen binding fragment of conventional antibodies
- Fc (Fragment crystallizable):
-
the antibody fragment composed of constant domains (CH2 and CH3) is responsible for effector functions
- VNAR:
-
variable domain of the shark new antigen receptor
- CDR (Complementarity Determining Regions):
-
hypervariable region of the variable antibody fragment
- FR (Framework Region):
-
conservative framework region of the variable antibody fragment.
References
Porter R.R. 1973. Structural studies of immunoglobulins. Science. 180, 713–716.
Padlan E.A. 1994. Anatomy of the antibody molecule. Mol. Immunol. 31, 169–217.
Dwek R.A., Sutton B.J., Perkins S.J., Rademacher T.W. 1984. Structure-function relationships in immunoglobulins. Biochem. Soc. Symp. 49, 123–136.
Burton D.R. 1985. Immunoglobulin G: Functional sites. Mol. Immunol. 22, 161–206.
Hamers-Casterman C., Atarhouch T., Muyldermans S., Robinson G., Hamers C., Bajyana Songa E., Bendahman N., Hamers R. 1993. Naturally occurring antibodies devoid of light chains. Nature. 363, 446–448.
Greenberg A.S., Avila D., Hughes M., Hughes A., Mckinney E.C., Flajnik M.F. 1995. A new antigen receptor gene family that undergoes rearrangement and extensive somatic diversification in sharks. Nature. 374, 168–173.
Rast J.P., Amemiya C.T., Litman R.T., Strong S.J., Litman G.W. 1998. Distinct patterns of IgH structure and organization in divergent lineage of chondrichthyan fishes. Immunogenetics. 47, 234–245.
Nuttall S.D., Krishnan U.V., Hattarki M., De Gori R., Irving R.A., Hudson P.J. 2001. Isolation of a new antigen receptor from wobbegong sharks, and use as a scaffold for the display of protein loop libraries. Mol. Immunol. 38, 313–326.
Nguyen V.K., Hamers R., Wyns L., Muyldermans S. 1999. Loss of splice consensus signal is responsible for the removal of the entire CH1 domain of the functional camel IGG2A heavy-chain antibodies. Mol. Immunol. 36, 515–524.
Woolven B.P., Frenken L., van der Logt P., Nicholls P.J. 1999. The structure of the llama heavy chain constant genes reveals a mechanism for heavy-chain antibody formation. Immunogenetics. 50, 98–101.
Nguyen V.K., Hamers R., Wyns L., Muyldermans S. 2000. Camel heavy-chain antibodies: Diverse germline VHH and specific mechanisms enlarge the antigen-binding repertoire. EMBO J. 19, 921–931.
De Genst E., Saerens D., Muyldermans S., Conrath K. 2006. Antibody repertoire development in camelids. Dev. Comp. Immunol. 30, 187–198.
Muyldermans S., Cambillau C., Wyns L. 2001. Recognition of antigens by single-domain antibody fragments: the superfluous luxury of paired domains. Trends Biochem. Sci. 26, 230–235.
Padlan E.A. 1996. X-Ray crystallography of antibodies. Adv. Protein Chem. 49, 57–133.
De Genst E., Silence K., Decanniere K., Loris R., Kinne J., Muyldermans S. 2006. Molecular basis for the preferential cleft recognition by dromedary heavy-chain antibodies. Proc. Natl. Acad. Sci. U.S.A. 103, 4586–4591.
Kabat E., Wu T.T., Perry H.M., Gottesman K.S., Foeller C. 1991. Sequence of Proteins of Immunological Interest. US Public Health Services Publication no. 91-3242. Bethesda, MD.
Nguyen V.K., Desmyter A., Muyldermans S. 2001. Functional heavy-chain antibodies in camelidae. Adv. Immunol. 79, 261–296.
Ghassabeh G.H., Muyldermans S., Saerens D. 2010. Nanobodies, single-domain antigen-binding fragments of camelid heavy-chain antibodies. In: Current Trends in Monoclonal Antibody Development and Manufacturing. Eds. Shire S.J. et al. NY: Springer, 29–48.
Wesolowski J., Alzogaray V., Reyelt J., et al. 2009. Single domain antibodies: Promising experimental and therapeutic tools in infection and immunity. Med. Microbiol. Immunol. 198, 157–174.
Muyldermans S., Baral T.N., Retamozzo V.C., et al. 2009. Camelid immunoglobulins and nanobody technology. Vet. Immunol. Immunopathol. 128(1–3), 178–183.
de Genst E., Handelberg F., van Meirhaeghe A., Vinck S., Loris R., Wyns L., Muyldermans S. 2004. Chemical basis for the affinity maturation of a camel single domain antibody. J. Biol. Chem. 279, 53593–53601.
de Genst E., Silence K., Decanniere K., Loris R., Kinne J., Wyns L., Muyldermans S. 2005. Strong in vivo maturation compensates for structurally restricted H3 loops in antibody repertoires. J. Biol. Chem. 280, 14114–14121.
Decanniere K., Muyldermans S., Wyns L. 2000. Canonical antigen binding loop structures: More structures, more canonical classes? J. Mol. Biol. 300, 83–91.
Desmyter A., Transue T.R., Ghahroudi M., Dao-Thi M.-H., Poortmans F., Hamers R., Muyldermans S., Wyns L. 1996. Crystal structure of a camel single-domain VH antibody fragment in complex with lysozyme. Nature Struct. Biol. 3, 8003–8011.
Lauwereys M., Ghahroudi M., Desmyter A., Kinne J., Holzer W., De Genst E., Wyns L., Muyldermans S. 1998. Potent enzyme inhibitors derived from drome-dary heavy-chain antibodies. EMBO J. 17, 3512–3520.
Brissette R., Goldstein N.I. 2007. The use of phage display peptide libraries for basic and translational research. Methods Mol. Biol. 383, 203–213.
Sidhu S.S., Koide S. 2007. Phage display for engineering and analyzing protein interaction interfaces. Curr. Opin. Struct. Biol. 17, 481–487.
Hoogenboom H.R. 2005. Selecting and screening recombinant antibody libraries. Nature Biotechnol. 23, 1105–1116.
Ghahroudi M.A., Desmyter A., Wyns L., Hamers R., Muyldermans S. 1997. Selection and identification of single domain antibody fragments from camel heavy-chain antibodies. FEBS Lett. 414, 521–526.
Saerens D., Kinne J., Bosmans E., Wernery U., Muyldermans S., Conrath K. 2004. Single domain antibodies derived from dromedary lymph node and peripheral blood lymphocytes sensing conformational variants of prostate-specific antigen. J. Biol. Chem. 279, 51965–51972.
Yau K.Y., Groves M.A., Li S., Sheedly C., Lee H., Tanha J., MacKenzie C.R., Jermutus L., Hall J.C. 2003. Selection of hapten-specific single-domain anti-bodies from a non-immunized llama ribosome display library. J. Immunol. Methods. 281, 161–175.
Conrath K.E., Lauwereys M., Galleni M., Matagne A., Frere J.M., Kinne J., Wyns L., Muyldermans S. 2001. Beta-lactamase inhibitors derived from single-domain antibody fragments elicited in Camelidae. Antimicrob. Agents Chemother. 45, 2807–2812.
Vyatchanin A.S., Tillib S.V. 2008. Modifications in the phage display procedure to increase the selection efficiency of antigen-binding domains of single-chain camel antibodies. Biotekhnologiya. 4, 32–34.
Tillib S.V., Ivanova T.I., Vasilev L.A. 2010. Fingerprint-like analysis of “nanoantibody” selection by phage display method using two helper phage variants. Acta Naturae. 2, 3 (6), 100–108.
Harmsen M.M., Haad H.J. 2007. Properties, production, and applications of camelid single-domain antibody fragments. Appl. Microbiol. Biotechnol. 77(1), 13–22.
Vincke C., Loris R., Saerens D., Martinez-Rodriguez S., Muyldermans S., Conrath K. 2009. General strategy to humanize a camelid single-domain antibody and identification of a universal humanized nanobody scaffold. J. Biol. Chem. 284(5), 3273–3284.
Martin F., Volpari C., Steinkuhler C., Dimasi N., Brunetti M., Biasiol G., Altamura S., Cortese R., De Francesco R., Sollazzo M. 1997. Affinity selection of a camelized VH domain antibody inhibitor of hepatitis C virus NS3 protease. Protein Eng. 10, 607–614.
Koch-Nolte F., Reyelt J., Schlossow B., Schwarz N., Scheuplein F., Rothenburg S., Haag F., Alzogaray V., Cauerhff A., Goldbaum F.A. 2007. Single domain anti-bodies from llama effectively and specifically block T cell ecto-ADP-ribosyltransferase ART2.2 in vivo. FASEB J. 21, 3490–3498.
Klooster R., Maassen B.T.H., Stam J.C., Hermans P.W., ten Haaft M.R., Detmers F.JM., de Haard H.J., Post J.A., Verrips C.T. 2007. Improved anti-IgG and HAS affinity ligands: Clinical application of VHH antibody technology. J. Immunol. Meth. 324, 1–12.
Jobling S.A., Jarman C., Teh M.M., Holmberg N., Blake C., Verhoeyen M.E. 2003. Immunomodulation of enzyme function in plants by single-domain anti-body fragments. Nature Biotechnol. 21, 77–80.
Gueorguieva D., Li S., Walsh N., Mukerji A., Tanha J., Pandey S. 2006. Identification of single-domain, Baxspecific intrabodies that confer resistance to mammalian cells against oxidative-stress-induced apoptosis. FASEB J. 20, 2636–2638.
Rothbauer U., Zolghadr K., Tillib S., Nowak D., Schermelleh L., Gahl A., Backmann N., Conrath K., Muyldermans S., Cardoso M.C., Leonhardt L. 2006. Targeting and tracing antigens in live cells with fluorescent nanobodies. Nature Methods. 3, 887–889.
Verheesen P., de Kluijver A., van Koningsbruggen S., de Brij M., de Haard H., van Ommen C.J.B., van der Maarel S.M., Verrips T. 2006. Prevention of oculopharyngeal muscular dystrophy-associated aggregation of nuclear poly(A)-binding protein with a single-domain intracellular antibody. Hum. Mol. Genet. 15, 105–111.
Pleschberger M., Saerens D., Weigert S., Sleytr U.B., Muyldermans S., Sara M., Egelseer E.M. 2004. An S-layer heavy chain camel antibody fusion protein for generation of a nanopatterned sensing layer to detect the prostate-specific antigen by surface plasmon resonance technology. Bioconjug. Chem. 15, 664–671.
Huang L., Reekmans G., Saerens D., Friedt J.M., Frederix F., Francis L., Muyldermans S., Campitelly A., van Hoof C. 2005. Prostate-specific antigen immunosensing based on mixed self-assembled monolayers, camel antibodies and colloidal gold enhanced sandwich assays. Biosens. Bioelectron. 21, 483–490.
Saerens D., Frederix F., Reekmans G., Conrath K., Jans K., Brys L., Huang L., Bosmans E., Maes G., Borghs G., Muyldermans S. 2005. Engineering camel single-domain antibodies and immobilization chemistry for human prostate-specific antigen sensing. Anal. Chem. 77, 7547–7555.
Huang Y., Verheesen P., Roussis A., et al. 2005. Protein studies in dysferlinopathy patients using llama-derived antibody fragments selected by phagedisplay. Eur. J. Hum. Genet. 13, 721–730.
Vyatchanin A.S., Tillib S.V. 2008. A new approach to study cellular components associated with a certain protein. Dokl. Akad Nauk. 421, 235–238.
Cortez-Retamozo V., Backmann N., Senter P.D., Wernery U., de Baetselier P., Muyldermans S., Revets H. 2004. Efficient cancer therapy with a nanobody-based conjugate. Cancer Res. 64, 2853–2857.
Roovers R.C., Laeremans T., Huang L., de Taeye S., Verkleij A.J., Revets H., de Haard H.J., van Bergen en Henegouwen P.M.P. 2007. Efficient inhibition of EGFR signaling and of tumor growth by antagonistic anti-EGFR nanobodies. Cancer Immunol. Immunother. 56, 303–317.
Coppieters K., Dreier T., Silence K., de Haard H., Lauwereys M., Casteels P., Beirnaert E., Jonckheere H., van de Wiele C., Staelens L., et al. 2006. Formatted anti-tumor necrosis factor alpha VHH proteins derived from camelids show superior potency and targeting to inflamed joints in a murine model of collagen-induced arthritis. Arthritis Rheum. 54, 1856–1866.
Dumoulin M., Last A.M., Desmyter A., Decanniere K., Canet D., Larsson G., Spencer A., Archer D.B., Sasse J., et al. 2003. A camelid antibody fragment inhibits the formation of amyloid fibrils human lysozyme. Nature. 424, 783–788.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Original Russian Text © S.V. Tillib, 2011, published in Molekulyarnaya Biologiya, 2011, Vol. 45, No. 1, pp. 77–85.
Rights and permissions
About this article
Cite this article
Tillib, S.V. “Camel nanoantibody” is an efficient tool for research, diagnostics and therapy. Mol Biol 45, 66–73 (2011). https://doi.org/10.1134/S0026893311010134
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0026893311010134