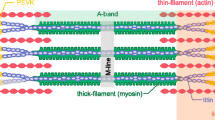
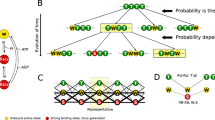
Abstract
To better understand the relationship between kinetic processes of contraction and the dynamic features of an isometric twitch, studies were conducted using a mathematical model that included: (1) kinetics of cross bridge (XB) cycling; (2) kinetics of thin filament regulatory processes; (3) serial and feedback interactions between these two kinetic processes; and (4) time course of calcium activation. Isometric twitch wave forms were predicted, morphometric features of the predicted twitch wave form were evaluated, and sensitivities of wave form morphometric features to model kinetic parameters were assessed. Initially, the impulse response of the XB cycle alone was analyzed with the findings that dynamic constants of the twitch transient were much faster than turnover number of steady-state XB cycling, and, although speed and duration of the twitch wave form were sensitive to XB cycle kinetic constants, parameters of wave shape were not. When thin filament regulatory unit (RU) kinetics were added to XB cycle kinetics, the system impulse response was slowed with only little effect on wave shape. When cooperative neighbor interactions between RU and XB were added, twitch wave shape (as well as amplitude, speed and duration) proved to be sensitive to variation in cooperativity. Importantly, persistence and shape of the falling phase could be strongly modified. When kinetic coefficients of XB attachment were made to depend on sarcomere length, changes in wave shape occurred that did not occur when only sliding filament mechanisms were operative. Indeed, the force–length relationship proved to be highly sensitive to length-dependent XB attachment in combination with cooperative interactions. These model findings are the basis of hypotheses for the role of specific kinetic events of contraction in generating twitch wave form features. © 2001 Biomedical Engineering Society.
PAC01: 8715La, 8719Rr, 8719Ff, 8710+e, 8717Aa, 8717-d, 8716Ka, 8715By
Similar content being viewed by others
REFERENCES
Barany, M. ATPase activity of myosin correlated with speed of muscle shortening. J.Gen.Physiol. 50:197–208, 1967.
Brandt, P. W., M. S. Diamond, J. S. Rutchik, and F. H. Sachat. Co-operative interactions between troponin-tropomyosin units extend the length of the thin filament in skeletal muscle. J.Mol.Biol. 195:885–896, 1987.
Brandt, P. W., M. S. Diamond, and F. H. Sachat. The thin filament of vertebrate skeletal muscle cooperatively activates as a unit. J.Mol.Biol. 180:379–384, 1984.
Bremel, R. D., and A. Weber. Cooperation within actin filament in vertebrate skeletal muscle. Nature (London), New Biol. 238:97–101, 1972.
Brenner, B. Correlation between the crossbridge cycle in muscle and actomyosin ATPase cycle in solution. J.Musc. Res. Cell Mot. 6:559–664, 1985.
Brenner, B. The cross-bridge cycle in muscle. Mechanical, biochemical, and structural studies on single kinetics muscle for correlation with the actomyosin-ATPase in solution. Basic Res.Cardiol. 81:1–15, 1986.
Campbell, K. Rate constant of muscle force redevelopment reflects cooperative activation as well as cross-bridge kinetics. Biophys.J. 72:254–262, 1997.
Campbell, K. B, M. Razumova, R. D. Kirkpatrick, and B. K. Slinker. Variably activated myofilament model for muscle contraction studies and applications. Thesis, Washington State University, Aug. 23, 2000, http:// people.vetmed.wsu.edu/KenCampbell.
Cecchi, G., and M. A. Bagni. Myofilament lattice spacing affects tension in striated muscle. News Physiol.Sci. 9:3–7, 1994.
Daniels, T. L., A. C. Trimble, and P. B. Chase. Compliant realignment of binding sites in muscle: Transient behavior and mechanical tuning. Biophys.J. 74:1611–1621, 1998.
Geeves, M. A., and S. S. Lehrer. Dynamics of the muscle thin filament regulatory switch: The size of the cooperative unit. Biophys.J. 67:273–282, 1994.
Godt, R. E., and D. W. Maughan. Influence of osmotic compression on calcium activation and tension in skinned muscle fibers of the rabbit. Pfluegers Arch. 391:334–337, 1981.
Goldman, Y. Kinetics of the actomyosin ATPase in muscle fibers. Annu.Rev.Physiol. 49:637–654, 1987.
Guth, K., K. J. V. Poole, D. Maughan, and H. J. Kuhn. The apparent rates of crossbridge attachment and detachment estimated from ATPase activity in insect flight muscle. Biophys.J. 52:1039–1045, 1987.
Hill, T. L. Cooperativity Theory in Biochemistry. New York: Springer, 1985.
Huxley, A. F. Muscle structure and theories of contraction. Prog.Biophys.Biophys.Chem. 7:255–318, 1957.
Jansen, P. M. L., and W. C. Hunter. Force, not length, correlates with prologation of isosarcomeric contraction. Am.J.Physiol. 269:H676–H685, 1995.
Razumova, M. V., A. E. Bukatina, and K. B. Campbell. Stiffness-distortion sarcomere model for muscle simulation. J.Appl.Physiol. 87:1861–1876, 1999.
Razumova, M. V., A. E. Bukatina, and K. B. Campbell. Different myofilament nearest-neighbor interactions have distinctive effects on contractile behavior. Biophys.J. 78:1320–1876, 2000.
Rome, L. C., and S. L. Lindstedt. The quest for speed: Muscles built for high-frequency contractions. News Physiol.Sci. 13:261–268, 1998.
Rome, L. C., D. A. Syme, S. Hollingworth, S. L. Lindstedt, and S. M. Baylor. The whistle and the rattle: the design of sound producing muscles. Proc.Natl.Acad.Sci.U.S.A. 93:8095–8100, 1996.
S. M. Selby, editor, CRC Standard Math Tables, 16th ed. Cleveland, OH: The Chemical Rubber Company, 1968.
Solaro, R. J., and H. M. Rarick. Troponin and tropomyosin: Proteins that switch on and tune in the activity of cardiac myofilaments. Circ.Res. 83:471–480, 1998.
Tobacman, L. S. Thin filament mediated regulation of cardiac contraction. Annu.Rev.Physiol. 58:447–481, 1996.
Tobias, A. H., B. K. Slinker, R. D. Kirkpatrick, and K. B. Campbell. Mechanical determinants of left ventricular relaxation. Am.J.Physiol. 268:H170–H177, 1995.
Wray, J. S. Filament geometry and the activation of insect flight muscle. Nature (London) 280:325–326, 1979.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Campbell, K.B., Razumova, M.V., Kirkpatrick, R.D. et al. Myofilament Kinetics in Isometric Twitch Dynamics. Annals of Biomedical Engineering 29, 384–405 (2001). https://doi.org/10.1114/1.1366669
Issue Date:
DOI: https://doi.org/10.1114/1.1366669