Abstract
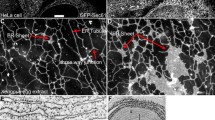
SH2/SH3 adaptor proteins are essential components of the signal transduction pathways initiated by tyrosine kinases. Nck is a ubiquitously expressed adaptor protein whose function has been enigmatic. We performed confocal microscopy to localize Nck in NIH3T3 and A431 cells. Surprisingly, Nck was identified in the nucleus as well as the cytoplasm with no visible change in localization due to PDGF or EGF stimulation. Western blot analysis of nuclear and cytosolic fractions confirmed that there was no translocation in response to growth factor and that tyrosine phosphorylation was specific to only cytosolic Nck. Far Western blot analysis with either Nck, the SH2 domain, or the SH3 domains revealed differential binding in nuclear and cytosolic lysates, indicating specific binding partners for each subcellular location. The major target of c-Src during mitosis is SAM68, a RNA-binding protein ordinarily localized to the nucleus. SAM68 was identified as a nuclear specific binding partner of Nck in both non-mitotic and mitotic cells. Several tyrosine kinases can be found in the nucleus but their signal transduction remains undefined. The discovery of an adaptor protein in the nucleus suggests there are signal transduction mechanisms within the nucleus that recapitulate those found in the cytoplasm.
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Lawe, D., Hahn, C. & Wong, A. The Nck SH2/SH3 adaptor protein is present in the nucleus and associates with the nuclear protein SAM68. Oncogene 14, 223–231 (1997). https://doi.org/10.1038/sj.onc.1200821
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/sj.onc.1200821
- Springer Nature Limited
Keywords
This article is cited by
-
Nck2 promotes human melanoma cell proliferation, migration and invasion in vitro and primary melanoma-derived tumor growth in vivo
BMC Cancer (2011)
-
Nck adapter proteins: functional versatility in T cells
Cell Communication and Signaling (2009)
-
Selected glimpses into the activation and function of Src kinase
Oncogene (2000)