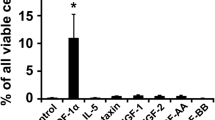
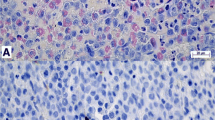
Abstract
Idiopathic hypereosinophilic syndromes (HES) comprise a spectrum of indolent to aggressive diseases characterized by persistent hypereosinophilia. Hypereosinophilia can result from the presence of a defect in the hematopoietic stem cell giving rise to eosinophilia, it can be present in many myeloproliferative disorders or alternatively it may be a reactive form, secondary to many clinical conditions. The hybrid gene FIP1L1-PDGRFα was identified in a subset of patients presenting with HES or chronic eosinophilic leukemia (CEL). In spite of this, the majority of HES patients do not present detectable molecular lesions and for many of them the diagnosis is based on exclusion criteria and sometimes it remains doubt. In this study we explored the possibility to distinguish between HES/CEL and reactive hypereosinophilia based on WT1 transcript amount. For this purpose, 312 patients with hypereosinophilia were characterized at the molecular and cytogenetic level and analyzed for WT1 expression at diagnosis and during follow-up. This study clearly demonstrates that WT1 quantitative assessment allows to discriminate between HES/CEL and reactive eosinophilia and represents a useful tool for disease monitoring especially in the patients lacking a marker of clonality.





Similar content being viewed by others
References
Bain BJ . Hypereosinophilia. Curr Opin Hematol 2000; 7: 21–25.
Weller PF, Bubley GJ . The idiopathic hypereosinophilic syndrome. Blood 1994; 83: 2759–2779.
Roufosse F, Cogan E, Goldman M . The hypereosinophilic syndrome revisited. Annu Rev Med 2003; 54: 169–184.
Vowels BR, Cassin M, Vonderheid EC, Rook AH . Aberrant cytokine production by Sezary syndrome patients: cytokine secretion pattern resembles murine Th2 cells. J Invest Dermatol 1992; 99: 90–94.
Simon HU, Plotz SG, Dummer R, Blaser K . Abnormal clones of T cells producing interleukin-5 in idiopathic eosinophilia. N Engl J Med 1999; 341: 1112–1120.
Luppi M, Marasca R, Morselli M, Brozzi P, Torelli G . Clonal nature of hypereosinophilic syndrome. Blood 1994; 84: 349–350.
Chang HW, Leong KH, Koh DR, Lee SH . Clonality of isolated eosinophils in the hypereosinophilic syndrome. Blood 1999; 93: 1651–1657.
Cools J, DeAngelo DJ, Gotlib J, Stover EH, Legare RD, Cortes J et al. A tyrosine kinase created by the fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med 2003; 348: 1201–1214.
Gleich GJ, Leiferman KM, Pardanani A, Tefferi A, Butterfield JH . Treatment of hypereosinophilic syndrome with imatinib mesilate. Lancet 2002; 359: 1577–1578.
Coutre S, Gotlib J . Targeted treatment of hypereosinophilic syndromes and chronic eosinophilic leukemias with imatinib mesylate. Semin Cancer Biol 2004; 4: 307–315.
Pardanani A, Brockman SR, Paternoster SF, Flynn HC, Ketterling RP, Lasho TL et al. FIP1L1-PDGFRA fusion: prevalence and clinicopathologic correlates in 89 consecutive patients with moderate to severe eosinophilia. Blood 2004; 104: 3038–3045.
Stone RM, Gilliland DG, Klion AD . Platelet-derived growth factor receptor inhibition to treat idiopathic hypereosinophilic syndrome. Semin Oncol 2004; 31: 12–17.
Gotlib J, Cools J, Malone JM, Schrier SL, Gilliland DJ, Coutre SE . The FIP1L1-PDGFRa fusion tyrosine kinase in hypereosinophilic syndrome and chronic eosinophilic leukemia: implications for diagnosis, classification, and management. Blood 2004; 103: 2879–2888.
Bigoni R, Cuneo A, Roberti MG, Dilani R, Bardi A, Cavazzini F et al. Cytogenetic and molecular cytogenetic characterization of 6 new cases of idiopathic hypereosinophilic syndrome. Haematologica 2000; 85: 486–491.
Hardy WR, Anderson RE . The hypereosinophilic syndromes. Ann Intern Med 1968; 68: 1220–1229.
Call KM, Glaser T, Ito CY, Buckler AJ, Pelletier J, Haber DA et al. Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms' tumor locus. Cell 1990; 60: 509–520.
Keilholz U, Menssen HD, Gaiger A, Menke A, Oji Y, Oka Y et al. Wilms' tumour gene 1 (WT1) in human neoplasia. Leukemia 2005; 19: 1318–1323.
Cilloni D, Gottardi E, Messa F, Fava M, Scaravaglio P, Bertini M et al. Significant correlation between the degree of WT1 expression level and the International Prognostic Scoring System Score in patients with myelodysplastic syndromes. J Clin Oncol 2003; 21: 1988–1995.
Inoue K, Ogawa H, Sonoda Y, Kimura T, sakabe H, Oka Y et al. Aberrant overexpression of the Wilms tumor gene (WT1) in human leukaemia. Blood 1997; 89: 1405–1412.
Cilloni D, Gottardi E, De Micheli D, Serra A, Volpe G, Messa F et al. Quantitative assessment of WT1 expression by real time quantitative PCR may be a useful tool for monitoring minimal residual disease in acute leukemia patients. Leukemia 2002; 16: 2115–2121.
Inoue K, Ogawa H, Yamagami T, Soma T, Tani Y, Tatekawa T et al. Long-term follow-up of minimal residual disease in leukemia patients by monitoring WT1 (Wilms tumor gene) expression levels. Blood 1996; 88: 2267–2278.
Cilloni D, Saglio G . Usefulness of quantitative assessment of Wilms tumor suppressor gene expression in chronic myeloid leukaemia patients undergoing Imatinib therapy. Semin Hematol 2003; 40: 37–41.
Tamaki H, Mishima M, Kawakami M, Tsuboi A, Kim EH, Hosen N et al. Monitoring minimal residual disease in leukemia using real-time quantitative polymerase chain reaction for Wilms tumor gene (WT1). Int J Hematol 2003; 78: 349–356.
Ogawa H, Tamaki H, Ikegame K, Soma T, Kawakami M, Tsuboi A et al. The usefulness of monitoring WT1 gene transcripts for the prediction and management of relapse following allogeneic stem cell transplantation in acute type leukemia. Blood 2003; 101: 1698–1704.
Bain B, Pierre R, Imbert M . Chronic eosinophilic leukemia and the hypereosinophilic syndrome. In: IARC (ed) World Health Organization Classification of Tumors: tumours of the hematopoietic and lymphoid tissues. International Agency for Research in Cancer (IARC) Press: Lyon, France, 2001.
Van Dongen JJ, Macintyre EA, Gabert JA, Delabesse E, Rossi V, Saglio G et al. Standardized RT-PCR analysis of fusion gene transcripts from chromosome aberrations in acute leukemia for detection of minimal residual disease. Report of the BIOMED-1 Concerted Action: investigation of minimal residual disease in acute leukemia. Leukemia 1999; 13: 1901–1928.
Cilloni D, Gottardi E, Saglio E . WT1 overexpression in acute myeloid leukemia and myelodysplastic syndromes. Myeloid Leukemia: Methods and Protocols 2005; 125: 199–211.
Tefferi A, Patnaik MA, Pardanani A . Eosinophilia: secondary, clonal and idiopathic. Br J Haematol 2006; 133: 468–492.
Musto P, Falcone A, Sanpaolo G, Bodenizza C, Perla G, Minervini MM et al. Heterogeneity of response to imatinib-mesylate (glivec) in patients with hypereosinophilic syndrome: implications for dosing and pathogenesis. Leuk Lymphoma 2004; 45: 1219–1222.
Mitelman F, Panani A, Brandt L . Isochromosome 17 in a case of eosinophilic leukaemia. An abnormality common to eosinophilic and neutrophilic cells. Scand J Haematol 1975; 14: 308–312.
Needleman SW, Mane SM, Gutheil JC, Kapil V, Heyman MR, Testa JR . Hypereosinophilic syndrome with evolution to myeloproliferative disorder: temporal relationship to lossof y chromosome and c-N-ras activation. Hematol Pathol 1990; 4: 149–155.
Sjoblom T, Boureux A, Ronnstrand L, Heldin CH, Ghysdael J, Ostman A . Characterization of the chronic myelomonocytic leukemia associated TEL-PDGF betaR fusion protein. Oncogene 1999; 18: 7055–7062.
Haber DA, Buckler AJ . WT1: a novel tumor suppressor gene inactivated in Wilms' tumor. New Biol 1992; 4: 97–106.
Haber DA, Housman DE . Role of the WT1 gene in Wilms' tumour. Cancer Surv 1992; 12: 105–117.
Baird PN, Simmons PJ . Expressin of the Wilms' tumor gene (WT1) in normal hemopoiesis. Exp Hematol 1997; 25: 312–320.
Tamaki H, Ogawa H, Ohyashiki K, Ohyashiki JH, Iwama H, Inoue H et al. The Wilms' tumor gene is a good marker for diagnosis of disease progression of myelodysplastic syndromes. Leukemia 1999; 13: 393–399.
Shichishima T, Okamoto M, Ikeda K, Kaneshige T, Sugiyama H, Terasawa T et al. HLA class II haplotype and quantitation of WT1 RNA in Japanese patients with paroxysmal nocturnal hemoglobinuria. Blood 2002; 100: 22–28.
Acknowledgements
This work was supported by grants from AIRC (Associazione Italiana per la Ricerca sul Cancro), MURST-COFIN, AIL (Associazione Italiana contro le Leucemie) and Regione Piemonte.
Author information
Authors and Affiliations
Consortia
Corresponding author
Appendix
Appendix
The following authors have actively participated in this study: Anna Serra, Enrico Bracco, Alessandro Morotti (Torino); Francesco Onida (Milano); Alessandro Levis, Massimo Pini (Alessandria); Tomasz Sacha (Cracow); Francesco Iuliano (Catanzaro); Marina Liberati (Perugia); Francesco Buccisano (Roma); Alberto Santagostino (Vercelli); Lucia Tornagli (Monza); Gianluca Gaidano (Novara); Gianpaolo Fra (Novara); Dario Ferrero (Torino); Massimo Negrini (Ferrara); Mariano Rocchi (Bari).
Rights and permissions
About this article
Cite this article
Cilloni, D., Messa, F., Martinelli, G. et al. WT1 transcript amount discriminates secondary or reactive eosinophilia from idiopathic hypereosinophilic syndrome or chronic eosinophilic leukemia. Leukemia 21, 1442–1450 (2007). https://doi.org/10.1038/sj.leu.2404670
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.leu.2404670
- Springer Nature Limited
Keywords
This article is cited by
-
Bone marrow WT1 levels at diagnosis, post-induction and post-intensification in adult de novo AML
Leukemia (2013)
-
Isolated molecular relapse in FIP1L1-PDGFRα hypereosinophilic syndrome after discontinuation and single weekly dose of imatinib: need of quantitative molecular procedures to modulate imatinib dose
Cancer Chemotherapy and Pharmacology (2009)