Abstract
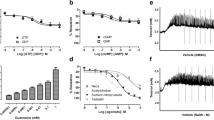
Constriction of the penile vasculature prevents erection and is largely mediated by physiological agonists. We hypothesized that protein kinase C (PKC) may act as a regulator of penile vascular tone. Studies were designed to identify PKC isoforms present and to investigate their roles in phenylephrine-induced muscle contraction in the isolated rat corpora cavernosa. We demonstrated the presence of PKCα, β, γ, ɛ, δ, η, and ι in rat corpora cavernosa and a subcellular distribution, which favored a membrane association for PKCα, β, δ, and ι. Phenylephrine (3 μM) generated an active stress of 9.6±1.5 mN/mm2 and was associated with a significant increase of PKCα and PKCι immunoreactivity in the particulate fraction. The amount of PKCα and PKCι in the particulate fraction rose by 36±4.4 and 51±2.2% with phenylephrine stimulation. Furthermore, the phenylephrine concentration–response curve was potentiated in the presence of phorbol 12-myristate13-acetate (PMA) (0.1 μM), a PKC activator (EC50: phenylephrine 1.0±0.8 μM vs phenylephrine+PMA 0.3±0.1 μM) and inhibited in the presence of chelerythrine chloride (30 μM), a PKC inhibitor (EC50: phenylephrine 1.0±0.8 μM vs phenylephrine+chelerythrine chloride 5.7±2.4 μM). Based on these results, we suggest a potential role for PKCα and PKCι in phenylephrine-induced smooth muscle tone of the rat cavernosum.






Similar content being viewed by others
References
Lue TF, Tanagho EA . Physiology of erection and pharmacological management of impotence. J Urol 1987; 137: 829–836.
Andersson KE . Pharmacology of penile erection. Pharmacol Rev 2001; 53: 417–450.
Gong MC, Fujihara H, Somlyo AV, Somlyo AP . Translocation of rhoA associated with Ca2+ sensitization of smooth muscle. J Biol Chem 1997; 272: 10704–10709.
Fujihara H et al. Inhibition of RhoA translocation and calcium sensitization by in vivo ADP-ribosylation with the chimeric toxin DC3B. Mol Biol Cell 1997; 8: 2437–2447.
Somlyo AP, Somlyo AV . Signal transduction and regulation in smooth muscle. Nature 1994; 372: 231–236.
Somlyo AP, Somlyo AV . Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol (Lond) 2000; 522(Part 2): 177–185.
Nishizuka Y . The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature 1988; 334: 661–665.
Somlyo AP et al. Inositol trisphosphate, calcium and muscle contraction. Phil Trans R Soc Lond B 1988; 320: 399–414.
Castagna M et al. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem 1982; 257: 7847–7851.
Rasmussen H, Takuwa Y, Park S . Protein kinase C in the regulation of smooth muscle contraction. FASEB J 1987; 1: 177–185.
Walsh MP et al. Smooth muscle protein kinase C. Can J Physiol Pharmacol 1994; 72: 1392–1399.
Nishizuka Y . Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science 1992; 258: 607–614.
Hug H, Sarre TF . Protein kinase C isoenzymes: divergence in signal transduction? Biochem J 1993; 291(Pt 2): 329–343.
Wingard CJ, Johnson JA, Holmes A, Prikosh A . Improved erectile function following Rho-kinase inhibition in a rat castrate model of erectile dysfunction. Am J Physiol Regul Integr Comp Physiol 2003; 284: R1572–R1579.
Husain S, Abdel-Latif AA . Role of protein kinase C alpha in endothelin-1 stimulation of cytosolic phospholipase A2 and arachidonic acid release in cultured cat iris sphincter smooth muscle cells. Biochim Biophys Acta 1998; 1392: 127–144.
Singer HA . Protein kinase C. In: Barany M (ed). Biochemistry of Smooth Muscle Contraction. Academic Press: San Diego, 1996, pp 155–165.
Husain S, Abdel-Latif AA . Protein kinase C isoforms in iris sphincter smooth muscle: differential effects of phorbol ester on contraction and cAMP accumulation are species specific. Curr Eye Res 1996; 15: 329–334.
Kraft AS, Anderson WB . Phorbol esters increase the amount of Ca2+, phospholipid-dependent protein kinase associated with plasma membrane. Nature 1983; 301: 621–623.
Selbie LA, Schmitz-Peiffer C, Sheng Y, Biden TJ . Molecular cloning and characterization of PKC iota, an atypical isoform of protein kinase C derived from insulin-secreting cells. J Biol Chem 1993; 268: 24296–24302.
Herbert JM, Augereau JM, Gleye J, Maffrand JP . Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun 1990; 172: 993–999.
O'brian CA, Kuo JF . Protin kinase C inhibitors. In: Kuo JF (ed). Protein Kinase C. Oxford University Press: New York, 1994, pp 96–120.
Quest AG, Bell RM . The molecular mechanism of protien kinase C regulation by lipids. In: Kuo JF (ed). Protein Kinase C. Oxford University Press: New York, 1994, pp 64–95.
Ryves WJ et al. Activation of the PKC-isotypes alpha, beta 1, gamma, delta and epsilon by phorbol esters of different biological activities. FEBS Lett 1991; 288: 5–9.
Karibe H, Oishi K, Uchida MK . Involvement of protein kinase C in Ca(2+)-independent contraction of rat uterine smooth muscle. Biochem Biophys Res Commun 1991; 179: 487–494.
Khalil RA, Lajoie C, Resnick MS, Morgan KG . Ca(2+)-independent isoforms of protein kinase C differentially translocate in smooth muscle. Am J Physiol 1992; 263: C714–C719.
Taggart MJ, Lee YH, Morgan KG . Cellular redistribution of PKCalpha, rhoA, and ROKalpha following smooth muscle agonist stimulation. Exp Cell Res 1999; 251: 92–101.
Lee YH et al. Isozyme-specific inhibitors of protein kinase C translocation: effects on contractility of single permeabilized vascular muscle cells of the ferret. J Physiol 1999; 517(Part 3): 709–720.
Ganz MB, Seftel A . Glucose-induced changes in protein kinase C and nitric oxide are prevented by vitamin E. Am J Physiol Endocrinol Metab 2000; 278: E146–E152.
Asaoka Y, Nakamura SI, Yoshida K, Nishizuka Y . Protein kinase C, calcium and phospholipid degradation. Trends Biochem Sci 1992; 17: 414–417.
Huang KP, Huang FL, Nakabayashi H, Yoshida Y . Biochemical characterization of rat brain protein kinase C isozymes. J Biol Chem 1988; 263: 14839–14845.
Li C, Xu Q . Mechanical stress-initiated signal transductions in vascular smooth muscle cells. Cell Signal 2000; 12: 435–445.
Williams B . Mechanical influences on vascular smooth muscle cell function. J Hypertens 1998; 16: 1921–1929.
Bauman AL, Scott JD . Kinase- and phosphatase-anchoring proteins: harnessing the dynamic duo. Nat Cell Biol 2002; 4: E203–E206.
Pawson T, Scott JD . Signaling through scaffold, anchoring, and adaptor proteins. Science 1997; 278: 2075–2080.
Michel JC, Scott JD . AKAP mediated signal transduction. Annu Rev Pharmacol Toxicol 2002; 42: 235–257.
Ron D, Kazanietz MG . New insights into the regulation of protein kinase C and novel phorbol ester receptors. FASEB J 1999; 13: 1658–1676.
Yoshida M et al. Effects of phorbol ester on lower urinary tract smooth muscles in rabbits. Eur J Pharmacol 1992; 222: 205–211.
Eto M et al. Histamine-induced vasoconstriction involves phosphorylation of a specific inhibitor protein for myosin phosphatase by protein kinase C alpha and delta isoforms. J Biol Chem 2001; 276: 29072–29078.
Murthy KS, Grider JR, Kuemmerle JF, Makhlouf GM . Sustained muscle contraction induced by agonists, growth factors, and Ca(2+) mediated by distinct PKC isozymes. Am J Physiol Gastrointest Liver Physiol 2000; 279: G201–G210.
Thieme H et al. Mediation of calcium-independent contraction in trabecular meshwork through protein kinase C and rho-A. Invest Ophthalmol Vis Sci 2000; 41: 4240–4246.
Bitar KN, Ibitayo A, Patil SB . HSP27 modulates agonist-induced association of translocated RhoA and PKC-alpha in muscle cells of the colon. J Appl Physiol 2002; 92: 41–49.
Ono Y et al. Protein kinase C zeta subspecies from rat brain: its structure, expression, and properties. Proc Natl Acad Sci USA 1989; 86: 3099–3103.
Nishizuka Y . Turnover of inositol phospholipids and signal transduction. Science 1984; 225: 1365–1370.
Osada S et al. A new member of the protein kinase C family, nPKC theta, predominantly expressed in skeletal muscle. Mol Cell Biol 1992; 12: 3930–3938.
Martiny-Baron G et al. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J Biol Chem 1993; 268: 9194–9197.
Toullec D et al. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem 1991; 266: 15771–15781.
Ikebe M, Brozovich FV . Protein kinase C increases force and slows relaxation in smooth muscle: evidence for regulation of the myosin light chain phosphatase. Biochem Biophys Res Commun 1996; 225: 370–376.
Gailly P, Gong MC, Somlyo AV, Somlyo AP . Possible role of atypical protein kinase C activated by arachidonic acid in Ca2+ sensitization of rabbit smooth muscle. J Physiol 1997; 500(Pt 1): 95–109.
Woodsome TP et al. Expression of CPI-17 and myosin phosphatase correlates with Ca(2+) sensitivity of protein kinase C-induced contraction in rabbit smooth muscle. J Physiol 2001; 535: 553–564.
Wang H et al. RhoA-mediated Ca2+ sensitization in erectile function. J Biol Chem 2002; 277: 30614–30621.
Hirata K et al. Involvement of rho p21 in the GTP-enhanced calcium ion sensitivity of smooth muscle contraction. J Biol Chem 1992; 267: 8719–8722.
Horowitz A, Menice CB, Laporte R, Morgan KG . Mechanisms of smooth muscle contraction. Physiol Rev 1996; 76: 967–1003.
Slater SJ, Seiz JL, Stagliano BA, Stubbs CD . Interaction of protein kinase C isozymes with Rho GTPases. Biochemistry 2001; 40: 4437–4445.
Damron DS et al. Role of PKC, tyrosine kinases, and Rho kinase in alpha-adrenoreceptor-mediated PASM contraction. Am J Physiol Lung Cell Mol Physiol 2002; 283: L1051–L1064.
Chitaley K et al. Antagonism of Rho-kinase stimulates rat penile erection via a nitric oxide-independent pathway. Nat Med 2001; 7: 119–122.
Rees RW et al. Y-27632, an inhibitor of Rho-kinase, antagonizes noradrenergic contractions in the rabbit and human penile corpus cavernosum. Br J Pharmacol 2001; 133: 455–458.
Acknowledgements
This work was supported by National Institute of Health (NIH DK59467) grant awarded to CJ Wingard. The authors are grateful to Dr John A Johnson for fruitful discussions during these studies.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Husain, S., Young, D. & Wingard, C. Role of PKCα and PKCι in phenylephrine-induced contraction of rat corpora cavernosa. Int J Impot Res 16, 325–333 (2004). https://doi.org/10.1038/sj.ijir.3901164
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijir.3901164
- Springer Nature Limited
Keywords
This article is cited by
-
Atorvastatin ameliorates the contractile dysfunction of the aorta induced by organ culture
Naunyn-Schmiedeberg's Archives of Pharmacology (2019)
-
Endothelial dysfunction in diabetic erectile dysfunction
International Journal of Impotence Research (2007)