Abstract
The prefrontal lobe has been considered to be closely related to depression. This study examined the relationship between depression and three prefronto-thalamic tract (PF-TT) regions (the dorsolateral prefronto-thalamic tract [DLPF-TT], ventrolateral prefronto-thalamic tract [VLPF-TT], and the orbitofronto-thalamic tract [OF-TT]) in patients with mild traumatic brain injury (TBI), using diffusion tensor tractography (DTT). Thirty-seven patients with depression following mild TBI were recruited based on Beck Depression Inventory-II (BDI-II) scores. Thirty-one normal control subjects were also recruited. The three regions of the PF-TTs were reconstructed using probabilistic tractography and DTT parameters for each of the three PF-TT regions were determined. The tract volume of the DLPF-TT and OF-TT in the patient group showed a significant decrease compared to that of the control group (p < 0.05). The BDI-II score of the patient group showed a moderate negative correlation with the tract volume value of the right (r = − 0.33) and left (r = − 0.41) DLPF-TT (p < 0.05). On the other hand, no significant correlations were detected between the BDI-II score of the patient group and the values of the other DTT parameters values for the three PF-TT regions (p > 0.05). Using DTT, depression was found to be closely related to a DLPF-TT injury in patients with mild TBI. We believe that evaluation of the DLPF-TT using DTT would be helpful when assessing patients with depression following mild TBI. These results can provide useful information regarding the proper application of neuromodulation in the management of depression.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Traumatic brain injury (TBI) may be classified into three types (mild, moderate, and severe) according to the level of injury severity1. Among these, mild TBI is observed in 75–90% of all TBI patients. Moreover, up to 44% of mild TBI patients were reported to suffer from depression2,3. Depression, a risk factor associated with poor recovery, leads to a range of problems, such as mood (anxiety, anger, and irritability), behavior (loss of interest and appetite and suicidal thinking), cognitive (memory and attention), physical (fatigue and headache), and sleep problems1,3. These problems can deteriorate a patient’s quality of life4. Therefore, it is important to investigate the pathogenic mechanism of depression. Previous studies related to the pathogenic mechanism of depression reported that depression is associated with multi-focal brain abnormalities including abnormalities in the cingulate cortex, amygdala, hippocampus, basal ganglia, thalamus, prefrontal cortex (PFC), and other brain regions5,6. In particular, the dorsolateral PFC (DLPFC), which manages the modulation and regulation of mood, is considered to play a key role in depression5,7,8. Many studies using various brain imaging methods, including positron emission tomography, functional magnetic resonance imaging (MRI), and voxel-based morphometry, have reported that injury of the DLPFC can lead to depression7,9,10,11,12,13,14,15,16. In addition, the DLPFC has been a primary treatment target region of repetitive transcranial magnetic stimulation used in the management of depression17,18.
Recent developments of diffusion tensor imaging (DTI), which measures the diffusion of water molecules, has allowed an investigation of the entire microstructural features of white matter in pre-defined regions of interest (ROIs)19. This also has the advantage of being able to detect even subtle changes of the white matter in the human brain19. DTI is suitable for use in an investigation of depression because depression is associated with various brain regions. Of particular note, diffusion tensor tractography (DTT), which is a derivative of DTI, provides for three-dimensional visualization and localization of neural tracts in the human brain19,20. Previously, three regions within the prefronto-thalamic tract (PF-TT), the dorsolateral prefronto-thalamic tract [DLPF-TT], the ventrolateral prefronto-thalamic tract [VLPF-TT] and the orbitofronto-thalamic tract [OF-TT] were reconstructed using DTT in normal subjects21. Many studies have used DTI to examine the changes in various brain regions in patients with depression, including the PFC, cingulate cortex, and amygdala8,11,12. Regarding TBI, many DTI studies of patients with post-traumatic stress disorder or depression have been reported22,23,24,25. Regarding the DTT, however, only one case study incorporated DTT in its investigation of depression in a patient with TBI26.
Mild TBI is not usually associated with lesions that can be detected by conventional brain magnetic resonance imaging (MRI)27. In contrast, since the introduction of DTI, many DTI-based studies could demonstrate axonal injury lesions that are related to the presence of various clinical features such as central pain, memory impairment, or motor weakness in mild TBI patients28,29. Regarding depression, one case study reported that a DLPF-TT injury was related to the presence of depression26. On the other hand, the regions within the PF-TT that may be related to depression in mild TBI have not demonstrated. This study hypothesized that, among three PF-TT regions, depression in patients with mild TBI would be associated with injury of the DLPF-TT.
In the current study, DTT was used to investigate the relationship between depression and tract injury in three PF-TT regions (DLPF-TT, VLPF-TT, and OF-TT) in patients with mild TBI.
Methods
Subjects
Fifty-eight patients with mild TBI visited the rehabilitation department of a university hospital (Table 1). Among the 58 patients (male: 20, female: 38, mean age: 47.3 ± 13.4 years, range: 13–68 years), thirty-seven (male: 12, female: 25, mean age: 45.7 ± 9.2 years, range: 23–58 years) were recruited according to the following inclusion criteria: (1) loss of consciousness for < 30 min, post-traumatic amnesia for ≤ 24 h, and an initial Glasgow Coma Scale score of 13–15; (2) no specific lesion observed on brain MRI (T1-weighted, T2-weighted, and fluid-attenuated inversion recovery images); (3) more than one month had elapsed after the onset of TBI; (4) age range of 20–59 years; (5) no history of previous head trauma, or neurological or psychiatric disease; (6) presence of depression on Beck Depression Inventory-II score (BDI-II, a full score of 63, a cutoff value of 13; a higher score means more severe depression)30. Values of BDI-II were obtained by self-report. In addition, 37 healthy control subjects (male: 12, female: 25, mean age: 45.6 ± 10.5 years, range: 24–59 years) with no previous history of neurological, physical, or psychiatric illness were recruited. There are no significant differences in sex, mean age, and whole brain volume between patient and control groups. All healthy subjects understood the purpose of the study and provided written, informed consent before participation. Because this study was conducted retrospectively, we could not obtain patient's consent and the study protocol without patient's consent was approved by the Institutional Review Board of a Yeungnam university hospital. (YUMC-2019-06-032). The study was carried out in accordance with relevant guidelines and regulations.
Diffusion tensor tractography
DTI data were acquired an average of 10.9 ± 11.5 months after the onset of head trauma using a six-channel head coil on a 1.5 T Philips Gyroscan Intera with 32 non-collinear diffusion sensitizing gradients (Philips, Ltd., Best, Netherlands). The imaging parameters were as follows: acquisition matrix = 96 × 96; reconstructed to matrix = 192 × 192; field of view = 240 × 240 mm2; repetition time = 10,398 ms; echo time = 72 ms; parallel imaging reduction factor (SENSE factor) = 2; echo-planar imaging factor = 59; b = 1000 s/mm2; and a slice thickness of 2.5 mm (acquired voxel size 1.25 × 1.25 × 2.5 mm3). The head motion effects and image distortions due to the eddy current were corrected by applying an affine multi-scale two-dimensional registration using Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (FSL; www.fmrib.ox.ac.uk/fsl)31. One analyzer (Kwon HG) performed fiber tracking using probabilistic tractography and applied the default tractography option (5000 streamline samples, 0.5 mm step lengths, curvature thresholds = 0.2) of the Oxford Centre for FMRIB Software Library with BedpostX method20,31,32. For reconstruction of the PF-TT regions a seed ROI was placed on the known anatomical location of the mediodorsal nucleus of the thalamus on the coronal image of the b0 map on each subject’s brain and each of the target ROIs as follows21: (1) DLPFC as Brodmann areas (BAs) 8, 9, and 46 on the coronal image of the b0 map; (2) ventrolateral PFC as BAs 44, 45, and 47 on the coronal image of the b0 map; and (3) orbitofrontal cortex as BAs 47/12, 10, 11, and 13 on the axial image of the b0 map. Values of fractional anisotropy (FA), mean diffusivity (MD), and tract volume (TV) were determined for each subject using MATLAB™ (Matlab R2007b, The Mathworks, Natick, MA, USA).
Statistical analysis
Statistical analyses were performed using SPSS software (v. 25.0; SPSS, Chicago, IL, USA). The independent t statistics were used to evaluate the differences between the DTT parameters (FA, MD, and TV values) of the three types of PF-TT regions (DLPF-TT, VLPF-TT, and OF-TT) in the patient and control groups. The r-statistic (Pearson correlation) was used to assess the relationships between the BDI-II score and the FA, MD, and TV values. The correlation coefficient (r) indicates the relative strength (0.1–0.3: weak, 0.3–0.5: moderate, > 0.5: strong) and direction (+ , −) of a linear relationship between the two values. The significance level of the obtained p value was set at 0.05.
Results
Table 2 presents a summary of the DTT parameters values for each of the three PF-TT regions as well as the correlations between the BDI-II score and DTT parameters of the three PF-TT regions (Table 2; Fig. 1). Regarding the DLPF-TT and OF-TT, the TV values of the patient group were significantly lower than those of the control group (p < 0.05). In contrast, there were no significant differences in the FA and MD values of the DLPF-TT and OF-TT between the patient and control groups (p > 0.05). In addition, there were no significant differences in the FA, MD, and TV values of the VLPF-TT between the patient and control groups (p > 0.05).
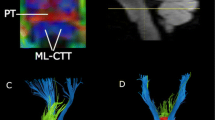
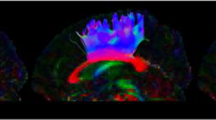
(A) T2-weighted brain magnetic resonance images showing no abnormalities in a representative patient (35-year-old male) and a normal subject (37-year-old male). (B) Results for the three prefronto-thalamic tract (PF-TT) regions on diffusion tensor tractography: DLPF-TT—dorsolateral prefronto-thalamic tract (red), VLPF-TT—ventrolateral prefronto-thalamic tract (green), and OF-TT—orbitofronto-thalamic tract (pink). The DLPF-TT in a patient shows partial tearing (green arrow) in the right hemisphere and narrowing (blue arrow) in the left hemisphere compared that that in a normal subject.
With regard to the correlation results, the BDI-II score of the patient group showed a moderate negative correlation with the TV values of the right (r = − 0.33) and left (r = − 0.41) DLPF-TTs (p < 0.05). On the other hand, no significant correlations were detected between the BDI-II score of the patient group and the other DTT parameter values of each of the three PF-TT regions (i.e., FA and MD of the DLPF-TT; FA, MD, and TV of the VLPF-TT and OF-TT) (p > 0.05).
Discussion
This study examined the relationship between depression and three regions of the PF-TT in patients with mild TBI. The results can be summarized as follows. (1) The TV values of the DLPF-TT and OF-TT in the patient group were lower than those of the control group. (2) The BDI-II score showed a moderate negative correlation with TV value of the DLPF-TT. Among the commonly assessed DTT parameters, the FA, MD, and TV have been used wildly to demonstrate the status of various neural tracts. The FA value indicates the degree of directionality of water diffusion. Consequently, it reflects the fiber density, axonal diameter, and white matter myelination, whereas the MD value indicates the magnitude of water diffusion33. In contrast, TV is a measure of the number of voxels contained within a neural tract. As a result, it is an indication of the number of neural fibers within a neural tract34. Therefore, the relatively low TV values in these patients were indicative of tract injuries of the DLPF-TT and OF-TT. Regarding the BDI-II correlation results, the results suggest that the severity of depression is closely related to the severity of the injury to the DLPF-TT, particularly TV.
Several studies have reported the presence of DLPFC injury in patients with depression7,9,10,11,12,13,14,15,16. These studies showed that the blood flow and gray matter volume of the DLPFC in patients with depression were lower than those in the control subjects7,9,10,13,14. Regarding DTI analyses, a few studies reported decreases in the FA value in the DLPFC area, which was measured using an ROI-based method, of patients with depression compared to those of the control subjects8,11,12. Specifically, Taylor et al. (2004) and Yang et al. (2007) demonstrated that the superior frontal gyrus including the DLPFC exhibited a lower FA value in patients with late-life depression than the normal subjects8,11. In addition, Blood et al. (2010) reported that DLPFC white matter in patients with major depressive disorder had a lower FA value compared to that of normal subjects12. With regard to DTT, only one case study has been reported. In 2016, Jang et al. reported the presence of DLPF-TT injuries in both hemispheres of a patient with depression26.
To the best of our knowledge, this is the first original study to demonstrate a relationship between depression and DLPF-TT injuries in patients with mild TBI by using DTT. However, some limitations of this study should be considered. First, only patients with depression who visited the rehabilitation department of a university hospital were recruited. Therefore, it is possible that among the patients with mild TBI, we recruited patients with a different level of severity of clinical manifestations than that of other patient populations. Second, regarding the results of significance of only TV (non-significance of FA and MD), we could not provide the apparent evidence why non-significance of FA and MD were shown. Third, their answer for BDI-II could have been exaggerating because it was self-reported. In addition, the generalizability of the results was affected by the spectrum bias, which refers to the differential performance of a test in different settings35. Fourth, although DTT is a powerful anatomic imaging tool that can demonstrate gross fiber architecture, it can produce false positive and false negative results due to crossing fibers or the partial volume effect36. Therefore, further studies will be needed to overcome the above limitations.
In conclusion, by using DTT, we observed that depression was closely related to the presence of DLPF-TT injury in patients with mild TBI. We believe that DTT-based evaluation of the DLPF-TT would be helpful for patients with depression following mild TBI. In addition, although these results can provide information that may be useful for determining the proper application of neuromodulation, such as repetitive transcranial magnetic stimulation or transcranial direct current stimulation, in the management of depression, TBI-related depression is different than from major depressive disorders in terms of the seizure risk37.
Data availability
The dataset used and/or analyzed during current study are available from the corresponding author on reasonable request.
References
Bhowmik, D., Sampath Kumar, K. P., Srivastava, S., Paswan, S. & Dutta, A. S. Depression—symptoms, causes. Medications and therapies. Pharma Innov. 1, 37–51 (2012).
Hoge, C. W. et al. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N. Engl. J. Med. 358, 453–463 (2008).
Fann, J. R., Uomoto, J. M. & Katon, W. J. Cognitive improvement with treatment of depression following mild traumatic brain injury. Psychosomatics 42, 48–54 (2001).
Sivertsen, H., Bjorklof, G. H., Engedal, K., Selbaek, G. & Helvik, A. S. Depression and quality of life in older persons: a review. Dement. Geriatr. Cogn. Disord. 40, 311–339 (2015).
Drevets, W. C. Neuroimaging studies of mood disorders. Biol. Psychiatry 48, 813–829 (2000).
Bora, E., Fornito, A., Pantelis, C. & Yucel, M. Gray matter abnormalities in major depressive disorder: a meta-analysis of voxel based morphometry studies. J. Affect. Disord. 138, 9–18 (2012).
Grieve, S. M., Korgaonkar, M. S., Koslow, S. H., Gordon, E. & Williams, L. M. Widespread reductions in gray matter volume in depression. Neuroimage Clin. 3, 332–339 (2013).
Taylor, W. D. et al. Late-life depression and microstructural abnormalities in dorsolateral prefrontal cortex white matter. Am. J. Psychiatry 161, 1293–1296 (2004).
Bench, C. J. et al. The anatomy of melancholia–focal abnormalities of cerebral blood flow in major depression. Psychol. Med. 22, 607–615 (1992).
Dolan, R. J. et al. Dorsolateral prefrontal cortex dysfunction in the major psychoses; symptom or disease specificity?. J. Neurol. Neurosurg. Psychiatry 56, 1290–1294 (1993).
Yang, Q., Huang, X., Hong, N. & Yu, X. White matter microstructural abnormalities in late-life depression. Int. Psychogeriatr. 19, 757–766 (2007).
Blood, A. J. et al. Microstructural abnormalities in subcortical reward circuitry of subjects with major depressive disorder. PLoS ONE 5, e13945 (2010).
Chang, C. C. et al. Reduction of dorsolateral prefrontal cortex gray matter in late-life depression. Psychiatry Res. 193, 1–6 (2011).
Lim, H. K. et al. Regional cortical thickness and subcortical volume changes are associated with cognitive impairments in the drug-naive patients with late-onset depression. Neuropsychopharmacology 37, 838–849 (2012).
Toenders, Y. J. et al. Neuroimaging predictors of onset and course of depression in childhood and adolescence: a systematic review of longitudinal studies. Dev. Cogn. Neurosci. 39, 100700 (2019).
Lopez, K. C., Luby, J. L., Belden, A. C. & Barch, D. M. Emotion dysregulation and functional connectivity in children with and without a history of major depressive disorder. Cogn. Affect. Behav. Neurosci. 18, 232–248 (2018).
Slotema, C. W., Blom, J. D., Hoek, H. W. & Sommer, I. E. Should we expand the toolbox of psychiatric treatment methods to include Repetitive Transcranial Magnetic Stimulation (rTMS)? A meta-analysis of the efficacy of rTMS in psychiatric disorders. J. Clin. Psychiatry 71, 873–884 (2010).
Martin, D. M., McClintock, S. M., Forster, J. J., Lo, T. Y. & Loo, C. K. Cognitive enhancing effects of rTMS administered to the prefrontal cortex in patients with depression: a systematic review and meta-analysis of individual task effects. Depress. Anxiety 34, 1029–1039 (2017).
Mori, S., Crain, B. J., Chacko, V. P. & van Zijl, P. C. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann. Neurol. 45, 265–269 (1999).
Behrens, T. E., Berg, H. J., Jbabdi, S., Rushworth, M. F. & Woolrich, M. W. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain?. Neuroimage 34, 144–155 (2007).
Jang, S. H. & Yeo, S. S. Thalamocortical connections between the mediodorsal nucleus of the thalamus and prefrontal cortex in the human brain: a diffusion tensor tractographic study. Yonsei Med. J. 55, 709–714 (2014).
Yeh, P. H. et al. Postconcussional disorder and PTSD symptoms of military-related traumatic brain injury associated with compromised neurocircuitry. Hum. Brain Mapp. 35, 2652–2673 (2014).
Main, K. L. et al. DTI measures identify mild and moderate TBI cases among patients with complex health problems: a receiver operating characteristic analysis of U.S. veterans. NeuroImage Clin 16, 1–16 (2017).
Gordon, E. M. et al. High-fidelity measures of whole-brain functional connectivity and white matter integrity mediate relationships between traumatic brain injury and post-traumatic stress disorder symptoms. J. Neurotrauma 35, 767–779 (2018).
Maller, J. J. et al. Traumatic brain injury, major depression, and diffusion tensor imaging: making connections. Brain Res. Rev. 64, 213–240 (2010).
Jang, S. H., Yi, J. H. & Kwon, H. G. Injury of the dorsolateral prefronto-thalamic tract in a patient with depression following mild traumatic brain injury: a case report. Medicine (Baltimore) 95, e5009 (2016).
Alexander, M. P. Mild traumatic brain injury: pathophysiology, natural history, and clinical management. Neurology 45, 1253–1260 (1995).
Shenton, M. E. et al. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav. 6, 137–192 (2012).
Jang, S. H. Traumatic Brain Injury (InTech, London, 2018).
Beck, A. T., Steer, R. A., Ball, R. & Ranieri, W. Comparison of Beck Depression Inventories-IA and -II in psychiatric outpatients. J. Pers. Assess. 67, 588–597 (1996).
Smith, S. M. et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23(Suppl 1), S208–S219 (2004).
Behrens, T. E. et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat. Neurosci. 6, 750–757 (2003).
Assaf, Y. & Pasternak, O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J. Mol. Neurosci. 34, 51–61 (2008).
Pagani, E., Agosta, F., Rocca, M. A., Caputo, D. & Filippi, M. Voxel-based analysis derived from fractional anisotropy images of white matter volume changes with aging. Neuroimage 41, 657–667 (2008).
Wang, Y. P. & Gorenstein, C. Assessment of depression in medical patients: a systematic review of the utility of the Beck Depression Inventory-II. Clinics (Sao Paulo) 68, 1274–1287 (2013).
Yamada, K., Sakai, K., Akazawa, K., Yuen, S. & Nishimura, T. MR tractography: a review of its clinical applications. Magn. Reson. Med. Sci. 8, 165–174 (2009).
Siddiqi, S. H. et al. Individualized connectome-targeted transcranial magnetic stimulation for neuropsychiatric sequelae of repetitive traumatic brain injury in a retired NFL player. J. Neuropsychiatry Clin. Neurosci. 31, 254–263. https://doi.org/10.1176/appi.neuropsych.18100230 (2019).
Acknowledgements
This work was supported by the Medical Research Center Program (2015R1A5A2009124) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning.
Author information
Authors and Affiliations
Contributions
S.H.J.: conceiving and designing the study, manuscript writing, data acquisition. H.G.K.: manuscript development, data analysis, manuscript writing and manuscript authorization.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jang, S.H., Kwon, H.G. Relationship between depression and dorsolateral prefronto-thalamic tract injury in patients with mild traumatic brain injury. Sci Rep 10, 19728 (2020). https://doi.org/10.1038/s41598-020-76889-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-76889-3
- Springer Nature Limited