Abstract
We report fabrication of new generation nanoflowers (NFs) using gallic acid (GA) and copper (II) ions (Cu2+) acted as an organic and inorganic component, respectively with effective peroxidase mimic activities in solution and on filter membrane. Unlike the typical protein NFs synthesis mechanism, gallic acid NFs (GA-NFs) was formed via coordination reaction between carboxyl groups of GA and Cu2+. The different morphologies of the GA-NFs were acquired based upon whether the carboxyl groups in gallic acid are active or not. The peroxidase mimic activity of the GA-NFs relied on the Fenton reaction in the presence of hydrogen peroxide (H2O2) was tested towards m-cresol as a function of concentration of the GA-NFs, m-cresol, H2O2 and reaction time. Under the optimized conditions, the oxidative coupling of m-cresol with 4-aminoantipyrine (4-AAP) was catalyzed by the GA-NFs dispersed in solution and adsorbed on filter paper to form an antipyrine dye and it was visually and spectrophotometrically recorded. The m-cresol with range of 0.05–0.5 mM was detected in 10 min and 15 min by using the GA-NFs in solution and on filter paper, respectively. We demonstrated that the NFs can be produced from non-protein molecules and GA-NFs can be used as a promising nanocatalyst for a variety of applications.
Similar content being viewed by others
Introduction
Recently, enzyme-inorganic flower-shaped hybrid nanostructures called “nanoflower (NF)” have received considerable attention owing to their greatly enhanced catalytic activity and stability compared to free and conventionally immobilized enzymes. Reasons for elevated activity and stability of the enzyme NFs can be: (1) high surface area, (2) alleviated mass-transfer limitations, (3) the increase in local concentration of the enzymes and (4) morphology dependent cooperative effect. The typical formation mechanism of proteins/enzymes-based NFs relies on forming complexes between amide groups of proteins/enzymes backbone and Cu2+ ions in phosphate buffer solution (PBS). Initially, Cu2+ ions reacted with phosphate ions (PO43−) to form Cu3(PO4)2 primary crystals, then, protein molecules bind to Cu3(PO4)2 primary crystals through coordination reaction between amide group and Cu2+ to from protein-Cu3(PO4)2 complexes as seeds, which can be called “ nucleation step”. The continuous feeding of Cu3(PO4)2 with protein results in seed growth for formation large petals and these petals are combined each other leading to multi-branched flower like structures via anisotropic growth, which can be called “growth step”1,2,3,4,5,6. Finally, the growth of nanoflowers reaches to saturation and their morphology is completed, which can be called “completion step”. Up to now, various enzymes-based NFs have been produced and utilized in many bioanalytical applications7,8,9,10,11,12. For instance, Wang and coworkers reported the synthesis of glucose oxidase (GOx) and horseradish peroxidase (HRP)-Cu3(PO4)2 hybrid NF for detection of glucose as a colorimetric sensor9. Additionally, Lu and coworkers developed a portable test kit prepared with acetylcholinesterase-NF incorporated agarose hydrogel for visual detection of acetylcholine10.
Moreover, while DNA capped gold or iron oxide nanoparticles (NPs) exhibited intrinsic peroxidase-like activity as DNA and colloidal NPs combination13,14, recent studies have shown that the amino acids, catecholamine and plant extracts can act as organic components and Cu2+ ions used as inorganic components for fabrication of the NFs instead using proteins/enzymes as organic molecules12,15,16. It is worthy to mention that amine and/or carboxyl groups in biological or organic molecules also coordinately react with Cu2+ ions to build up non-protein NFs. They have exhibited peroxidase like and antimicrobial activities benefiting from Fenton reaction mechanism in the presence of hydrogen peroxide (H2O2). However, no synthesis of NFs using amine group free molecules has been reported and formation mechanism has not been documented yet.
Phenol and its derivatives are industrial by product used in many processes, such as the production of pesticides, insecticides, plastics and dyes and have serious negative effects to human and to the environment17. Therefore, the development of analytical methods for determination of these compounds is of great importance. The several approaches or methods have been developed for analyzing these compounds including chromatographic18 and electrochemical methods19. However, these methods require expensive instruments, multi-step and time-consuming procedures and trained personnel as well. Colorimetric sensing has drawn attention due to low cost, easy to operate, one-step and rapid procedure for detection of phenolic compounds20.
Herein, we report, for the first time, the synthesis of gallic acid-based NFs (GA-NFs) with effective peroxidase mimic catalytic activity for colorimetric and spectrophotometric detection of m-cresol. The GA-NFs exhibited peroxidase mimic activity in the presence of H2O2 based on Fenton reaction. Basically, GA-NFs catalyzed oxidation of series of concentrations (0.05 mM, 0.1 mM, 0.2 mM, 0.3 mM, 0.4 mM, 0.5 mM) of m-cresol with of 4-AAP in solution within 10 minute (min) incubation and the products were spectrophotometrically and visually detected. Additionally, the same procedure was applied to GA-NFs immobilized filter membrane, then the detection of m-cresol with same concentrations were accomplished in 15 min. Various parameters such as concentration of GA-NFs, H2O2 and reaction time were investigated to optimize the detection of m-cresol in solution and on filter membrane.
Results
Synthesis of GA-NFs
The synthesis of organic–inorganic nanoflowers (NFs) relies on coordination reaction between amide groups in the protein backbone and Cu2+ in Cu3(PO4)2 primary crystals. Although, non-protein molecules including amino acids (known as the building blocks of proteins), catecholamines or some model plants extracts acted as organic components of the NFs, almost all recent studies have taken the coordination between the amide group and Cu2+ as a key step for formation of the NFs. It seems that the selection of amide group containing molecules have become a mandatory in NFs synthesis, which can be considered as a major disadvantage of the NFs formation.
Herein, we present, for the first time, an inspirational work with fabrication of gallic acid incorporated nanoflower (GA-NFs) by exploiting coordination reaction occurred between carboxyl group of GA and Cu2+. The typical NFs synthesis procedure was applied that free GA was added into 10 mM PBS solution containing Cu2+, and then the resulting mixture was left for incubation without disturbing. The carboxyl group of GA reacted with Cu2+ in Cu3(PO4)2 primary crystals to initiate the formation of GA-Cu3(PO4)2 complexes as seeds. Interestingly, GA-Cu3(PO4)2 complexes were grown as large petals and these GA-incorporated petals bound to each other by acting as a glue, as protein incorporated ones acted in discovery of NFs before. Then, complete flower-shaped structure called “nanoflower (NF)” is occurred with saturation of anisotropic growth. The formation mechanism of GA-NFs was demonstrated in Scheme 1 step by step.
As an interesting and worthy approach, the roles of activation of carboxyl group on the morphologies of GA-NFs were systematically examined. The 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysuccinimide (NHS) in standard protein labeling chemistry or called “EDC/NHS chemistry” was utilized to activate the carboxyl group of free GA. Then, typical NF synthesis procedure was followed to show how activated carboxyl group influence morphology of GA-NFs.
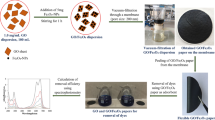
Structure of the GA-NFs with peroxidase mimic activities in solution and on filter membrane were characterized and interpreted. In our system, the GA-NFs were dispersed in solution and physically adsorbed on filter membrane for detection of m-cresol known as important hazardous compound, by UV–Vis spectrophotometer and naked eye (Fig. 1A). The oxidative coupling reaction between m-cresol and 4-AAP catalyzed by GA-NFs in the presence of H2O2 was shown in Fig. 1B.
Characterization of GA-NFs
The structure of GA-NFs was characterized via several methods. The morphologies (shape, size and surface property) of GA-NFs and carboxyl group activated GA-NFs (cGA-NFs) were monitored by scanning electron microscopy (SEM). The SEM images in Fig. 2A, B show that GA-NFs are spherical with ~ 4 µm size. It seems that the GA-incorporated large petals have plate like shapes, and they have vertically inserted each other to form the GA-NFs. The small spheres (shown in black square) on surface of magnified GA-NFs image in Fig. 2B can be indication of newly occurred GA-Cu3(PO4)2 nanocrystals. We hypothesize that formation of the GA-NFs may be kinetically slow and under continuous formation process. Figure 2C shows that when reaction time was prolonged, the small spheres grew and wrapped surface of the GA-NFs as belts (shown in black rectangular). With the worthy approach, we activated carboxyl group of GA to facilitate coordination reaction between GA and Cu2+. And then, the ~ 9 µm sized, uniform, and mono-dispersed cGA-NFs with highly porous structure were produced as shown in Fig. 2D. The high-magnification image of the cGA-NFs was also presented in inset of Fig. 2D.
As a further structural analysis, Energy Dispersive X-ray Analysis (EDX) was used to show the presence of Cu metal in the Cu3(PO4)2 scaffold (Fig. 3A). As the formation mechanism of the NF have been well documented, metal ions, especially Cu2+ acts as an indispensable cornerstone component for building of the NF. The bending and stretching in the cGA-NFs were evaluated with Fourier-transform infrared spectroscopy (FTIR) as shown in Fig. 3B. For analysis of free GA (blue line), the stretching of O–H groups at 3268 cm-1, strong absorption of COOH (carboxylic acids) at 1606 cm-1 and C=O stretching at 1467 cm-1 are attributed to characteristic peaks of free GA. The characteristic peaks of 1029 cm-1 and 555 cm-1 refer to PO43− vibrations of Cu3(PO4)2 as given with red line15. While the PO43− vibrations in cGA-NFs are assigned to 1038 cm-1 and 558 cm-1, the stretching of O–H group with different mode, moderate absorption of COOH and C=O stretching are observed at 3419 cm-1, 1623 cm-1, 1469 cm-1, respectively. The consistency in the FTIR spectra is an indication of cGA incorporated NFs. The diffraction peak positions of cGA-NFs spectrum show that the crystal pattern of Cu3(PO4)2·3H2O (JPSCD 00–022-0548) and NaCl (JPSCD 01-088-2300) can be both seen in XRD spectrum (Fig. 3C).
Detection of m-cresol
The m-cresol and its s isomers o-cresol and p-cresol can be enzymatically oxidized but with different efficiencies owing to favorable position of ring substituents in their structures21,22. We chose m-cresol for catalytic reaction due to high substrate specificity at room temperature (RT: 20 °C). After the synthesis of cGA-NFs, we demonstrated carboxyl group activated cGA-NFs exhibited much enhanced peroxidase mimic activity compared to GA-NFs formed of GA molecule containing non-activate carboxyl group. The reasons for morphology dependent activity can be attributed to highly porous and compact structures of cGA-NFs. As shown in Figure S1A, the cGA-NFs display higher peroxidase like activity to catalyze the reaction between 4-AAP and m-cresol in the presence of H2O2 than GA-NFs. Then, we only used cGA-NFs in all peroxidase mimic activity experiments. The cGA-NFs acted as a Fenton reagent in the presence of H2O2 and then exhibited peroxidase mimic activity through Fenton reaction. The potential mechanism for Fenton reaction is that Cu2+ ions in the cGA-NFs react with H2O2 to produce Cu1+. Followingly, interaction between Cu1+ and H2O2 resulted in highly reactive hydroxyl radical (·OH), which catalyzes oxidative coupling reaction between m-cresol and 4-AAP to form an antipyrine dye as a colored compound. The mechanism for the potential Fenton-like reaction is given in Eq. (1).
The peroxidase mimic activities of the cGA-NFs were evaluated in solution and on filter membrane against m-cresol under the various experimental parameters. In Fig. 4, the peroxidase mimic activity of the cGA-NFs dispersed in solution was studied. A standard activity protocol was followed; oxidative coupling between m-cresol and 4-AAP was catalyzed by the cGA-NFs with intrinsic peroxidase-mimic activity. The cGA-NFs (0.5 mg/mL) was added into solutions containing 4 mM 4-AAP, 1 mM H2O2 and m-cresol with a series of concentrations (0.05 mM, 0.1 mM, 0.2 mM, 0.3 mM, 0.4 mM, 0.5 mM) and each solution was incubated for 10 min, then its activity was tested in each solution as a function of m-cresol concentrations (Fig. 4A). It reveals that absorption values and color intensity (the direction of the arrow in the photo is from high concentration to low in Fig. 4A) of the product solutions were increased with increase in m-cresol concentrations. The same activity protocol in Fig. 4A was used; activity of the cGA-NFs was evaluated towards m-cresol fixed to 0.4 mM for different incubation times. 0.4 mM m-cresol, the cGA-NFs (0.5 mg/mL), 4 mM 4-AAP and 1 mM H2O2 were mixed together and incubated for 10 min, 30 min, 60 min and 90 min. The absorbance of each solution was measured by UV–Vis. Figure 4B shows that although intense color change and high absorbance values after catalytic reactions was obtained in long incubation times (90 min and 60 min), in 10 min or even in 5 min (data now shown) the remarkable color change and absorption increase were observed (the direction of the arrow in the photo is from high incubation time to short in Fig. 4B). The effect of presence of H2O2 was shown in Supplementary Information Figure S2. It reveals that the efficiency of oxidative coupling reaction between 4-AAP and m-cresol was enhanced with H2O2. To demonstrate the activity and stability of the cGA-NFs, the reaction solution containing 0.5 mg/mL cGA-NFs, 0.4 mM m-cresol, 4-AAP and 1 mM H2O2, was applied successive catalytic use (Fig. 4C). We clearly showed that the cGA-NFs lost 60% its initial catalytic activity over the six cycles, which may exhibit high catalytic performance and stability. SEM images of the GA-NFs were recorded before reaction (left one in Fig. 4D) and after the six cycles (right one in Fig. 4D). It is clear that the morphology of the GA-NFs was slightly distorted after six cycles in use compared to intact cGA-NFs image.
To use the cGA-NFs as an attractive nanobiocatalyst, we non-covalently deposited the cGA-NFs on the surface of commercial filter membrane, then investigate how it exhibit peroxidase mimic activity as function the cGA-NFs, m-cresol and H2O2 concentration and reaction time. As a first parameter (Fig. 5A), a series concentration of the cGA-NFs was absorbed on filter membranes, then reaction solution (4 mM 4-AAP, 1 mM H2O2 and 0.4 mM m-cresol) was injected each membrane to observe its activity in 15 min. It is noticed that using the filter membrane high amount of the cGA-NFs exhibited much efficient catalytic activity, which is quite consistent with absorption value and color intensity of the product solution (the direction of the arrow in the photo is from low amount cGA-NFs to high on filter membrane in Fig. 5A). We realized that 2 mg/mL cGA-NFs adsorbed filter membrane can be ideal nanobiocatalyst for further reaction owing to effective peroxidase mimic activity. As aforementioned above, presence of H2O2 and its concentration are vitally important for rapid and efficient catalytic activity as shown in Fig. 5B. We fixed the concentration of the cGA-NFs on filter membrane, m-cresol and 4-AAP to be 2 mg/mL, 0.4 mM and 4 mM, respectively, then concentrations of H2O2 were varied. Although we do not expect any catalytic activity in the absence of H2O2, the oxidation of m-cresol was carried out catalyzed by the cGA-NFs adsorbed filter membrane as demonstrated with absorption value and color change. We hypothesize that Cu2+ ion in the cGA-NFs react with m-cresol to give complexation reaction for formation of a chelate, then Cu2+ is reduced to Cu1+and oxidative coupling reaction of m-cresol with 4-AAP is occurred under the redox reaction. Interestingly, the filter membrane exhibited the highest catalytic activity in both 40 mM and 24 mM H2O2 as absorption values and color intensity of the product solution dictate this phenomenon (the direction of the arrow in the photo is from low concentration of H2O2 to high in Fig. 5B). After determining the ideal concentration of the cGA-NFs and H2O2, the effect of the m-cresol concentration was examined. Figure 5C shows that while even lowest concentration of m-cresol (0.05 mM) was oxidized by the filter membrane and spectrophotometrically and visually detected but in 60 min, however, almost the same catalytic performance was obtained when using 0.5 mM and 0.4 mM m-cresol. The recycling of the filter membrane was tested over the six catalytic cycles, the filter membrane maintained almost 60% of its first cycle activity even after six cycles (Fig. 5D). We hypothesize that favorable conformation of the cGA-NFs can be slightly changed after third cycle was, then gradual reduction in catalytic activities were observed after third cycle. And, the distance between petals or layers of cGA-NFs can decrease and they may stick each other, then catalytic activity can be adversely influenced after third cycle wash. We claim that the cGA-NFs adsorbed on filter membrane possessed much durability compared to the cGA-NFs dispersed in solution, as how the enzyme NFs show enhanced stability compared to free enzymes. We also monitored how successive catalytic reactions influence the morphologies of the cGA-NF on filter membrane with SEM images. The SEM image of the filter membrane were obtained after first and six cycles as seen on top of blue column (image after first cycle) and orange column (image after six cycles), we analyzed that no remarkable distortion on both SEM images. The potential reasons for reduction in catalytic activity of the filter membrane after repeated use can be i)adsorption of excess m-cresol on surface of the cGA-NFs may increase mass-transfer limitations and ii) excess m-cresol may attack available or accessible Cu2+ ions and form m-cresol-Cu2+ complexes, both of which may obstruct catalytic activity performance of the NFs. it is worthy to point out that although the morphology of the NFs is not significantly altered or impaired, the catalytic activity decreases due to the reasons mentioned above.
Images of the GA-NFs formed in (A, B) 3 days, (C) 4 days and (D) image of the carboxyl group activated cGA-NFs formed in 3 days. Inset: high-magnification image of Fig. 2D.
Discussion
In summary, we have developed cGA-NFs as new generation NFs and investigate their peroxidase mimic activities through the Fenton reaction when dispersed in solution and adsorbed on filter membrane towards m-cresol. In synthesis procedure, carboxyl group of GA molecules reacted with Cu2+ for formation of cGA-Cu3(PO4)2 primary nanocrystals, then cGA-NFs was kinetically formed. We also activated carboxyl group of GA, then we showed how it influences morphology and peroxidase mimic activity of the cGA-NFs. The peroxidase-mimic activities of the cGA-NFs in solution and on filter membrane was optimized under various experimental parameters. The both synthesis of the cGA-NFs and the preparation of cGA-NFs deposited filter membrane opened up new avenue in designing novel biocatalytic system. The uniform, mono-dispersed and porous cGA-NFs with intrinsic peroxidase-mimic activity can be promising alternative to enzyme-incorporated NFs and find widespread use in various scientific and technical fields.
Materials
Copper(II) sulfate pentahydrate (CuSO4·5H2O), m-cresol, gallic acid, 4-Aminoantipyrine (4-AAP), hydrogen peroxide (H2O2, 25% w/v) , salt precursor of PBS (NaCl, KCl, Na2HPO4, KH2PO4, CaCl2.2H2O, MgCl2.6H2O), 1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysuccinimide (NHS) were purchased from Sigma Aldrich. Cellulose acetate syringe filters (0.45 µm) were obtained from Isolab. All solutions were prepared with ultrapure water (resistance 18.2 MΩ). All chemicals were of analytical grade and used without further purification.
Methods
UV–Vis spectrophotometry (Shimadzu UV1800) was used for determination of peroxidase like activity of NFs. Scanning Electron Microscopy (SEM, ZEISS EVO LS10) was operated for imaging morphologies of NFs. IR spectra of NFs was recorded on a FT-IR (Thermo Scientific Nicolet 6700). Crystal structure of NFs and Cu3(PO4)2 primary crystal were analyzed by X-Ray Diffraction (XRD, Bruker AXS D8 Advance Model).
Formation of gallic acid nanoflowers
Organic inorganic nanoflowers were synthesized according to literature with some modifications16.Gallic acid was used as an organic part and Cu2+ ion acted as an inorganic part for the synthesis of GA-NF. Briefly, 0.02 mg/mL gallic acid was dissolved in distilled water. An aqueous solution of CuSO4 (120 mM, 660 µL) and gallic acid was added to 100 mL phosphate buffer saline (PBS) solution (10 mM, pH 7,4). The mixture was stirred vigorously for 5 min to increase interaction between Cu2+ and gallic acid. After the incubation at 25 °C for 3 days without disturbing, the precipitates formed at the bottom of the solution were collected through centrifugation (5000 rpm, 10 min) and washed with pure water several times. The obtained product was dried at 50 °C.
For the synthesis of cGA-NFs, 0.02 mg/mL was dissolved in PBS solution (10 mM, pH 7.4). The solution was mixed with EDC (10 mM) and NHS (12 mM), following by stirring overnight at room temperature. Then an aqueous solution of CuSO4 (120 mM, 660 µL) was added to mixture and incubated at 25 °C for 3 days. The obtained products bottom of the solution were collected through centrifugation (5000 rpm, 10 min) and washed with pure water several times. The cGA-NFs was dried at 50 °C.
Detection of m-cresol in solution
Different concentration of m-cresol (0.05 mM, 0.1 mM, 0.2 mM, 0.3 mM, 0.4 mM and 0.5 mM) was added to PBS solution (0,1 M pH 7,4) containing cGA-NFs (1 mg/mL), H2O2 and 4-AAP (4 mM). These solutions were incubated at room temperature for 15 min, followed by centrifugation at 8000 rpm for 5 min to remove cGA-NFs. The absorbance of resulting solutions was measured by UV–Vis spectrophotometer.
Detection of m-cresol on filter membrane
The GA-NF suspension in PBS was deposited on filter membrane (cellulose acetate membrane 0.45 µm) and air was pressed through the filter to remove PBS. Subsequently, the mixture containing different concentration of m-cresol, 4 mM 4-AAP and H2O2 was injected to filter with syringe. After incubation at room temperature for various periods of times on filter membrane, the product was collected into glass vial and the absorbance of the product was recorded by UV–Vis spectrophotometer.
References
Somturk, B., Hancer, M., Ocsoy, I. & Ozdemir, N. Synthesis of copper ion incorporated horseradish peroxidase-based hybrid nanoflowers for enhanced catalytic activity and stability. Dalton Trans. 44, 13845–13852 (2015).
Ocsoy, I., Dogru, E. & Usta, S. A new generation of flowerlike horseradish peroxides as a nanobiocatalyst for superior enzymatic activity. Enzyme Microb. Technol. 75–76, 25–29 (2015).
Somturk, B. et al. Synthesis of urease hybrid nanoflowers and their enhanced catalytic properties. Enzyme Microb. Technol. 86, 134–142 (2016).
Yilmaz, E., Ocsoy, I., Ozdemir, N. & Soylak, M. Bovine serum albumin-Cu(II) hybrid nanoflowers: an effective adsorbent for solid phase extraction and slurry sampling flame atomic absorption spectrometric analysis of cadmium and lead in water, hair, food and cigarette samples. Anal. Chim Acta 906, 110–117 (2016).
Altinkaynak, C. et al. Preparation of lactoperoxidase incorporated hybrid nanoflower and its excellent activity and stability. Int. J. Biol. Macromol. 84, 402–409 (2016).
Celik, C. et al. Formation of functional nanobiocatalysts with a novel and encouraging immobilization approach and their versatile bioanalytical applications. RSC Adv. 8, 25298–25303 (2018).
Ge, J., Lei, J. & Zare, R. N. Protein-inorganic nanoflowers. Nat. Nanotechnol. 7, 428–432 (2012).
Zhu, L. et al. Rapid detection of phenol using a membrane containing laccase nanoflowers. Chem. Asian J. 8, 2358–2360 (2013).
Sun, J. et al. Multi-enzyme co-embedded organic-inorganic hybrid nanoflowers: synthesis and application as a colorimetric sensor. Nanoscale 6, 255 (2014).
Kong, D. et al. Protein-inorganic hybrid nanoflower-rooted agarose hydrogel platform for point-of-care detection of acetylcholine. ACS Appl. Mater Interfaces 11(12), 11857–11864 (2019).
Wu, Z. et al. Amino acids-incorporated nanoflowers with an intrinsic peroxidase-like activity. Sci. Rep. 6, 22412 (2016).
Ildiz, N. et al. Self assembled snowball-like hybrid nanostructures comprising Viburnum opulus L. extract and metal ions for antimicrobial and catalytic applications. Enzyme Microb. Technol. 102, 60–66 (2017).
Liu, B. & Liu, J. Accelerating peroxidase mimicking nanozymes using DNA. Nanoscale 7, 13831–13835 (2015).
Hizir, M. S. et al. Multiplexed activity of perAuxidase: DNA-capped AuNPs act as adjustable peroxidase. Anal. Chem. 88, 600–605 (2016).
Baldemir, A. et al. Synthesis and characterization of green tea (Camellia sinensis (L) Kuntze) extract and its major components-based nanoflowers: a new strategy to enhance antimicrobial activity. RSC Adv. 7, 44303–44308 (2017).
Celik, C., Ildiz, N. & Ocsoy, I. Building block and rapid synthesis of catecholamines-inorganic nanoflowers with their peroxidase-mimicking and antimicrobial activities. Sci. Rep. 10, 2903–2020 (2020).
Alkasir, R., Ornatska, M. & Andreescu, S. Colorimetric paper bioassay for the detection of phenolic compounds. Anal. Chem. 84, 9729–9737 (2012).
Meng, J. R. et al. Preparation of Fe3O4@C@PANI magnetic microspheres for the extraction and analysis of phenolic compounds in water samples by gas chromatography-mass spectrometry. J. Chromatogr. A 1218, 2841–2847 (2011).
Liu, F., Piao, Y. X., Choi, J. S. & Seo, T. S. Three-dimensional graphene micropillar based electrochemical sensor for phenol detection. Biosens. Bioelectron. 50, 387–392 (2013).
Senyurt, O., Eyidogan, F. & Yilmaz, R. Development of a paper-type tyrosinase biosensor for detection of phenolic compounds. Biotechnol. Appl. Biochem. 62, 132–136 (2015).
Skoronski, E. et al. Substrate specificity and enzyme recycling using chitosan immobilized laccase. Molecules 19, 16794–16809 (2014).
Islam, M. S. & Harnett, C. K. Miniaturized systems for evaluating enzyme activity in polymeric membrane bioreactors. Eng. Life Sci. 19, 749–758 (2019).
Acknowledgements
We appreciate Erciyes University Technology Research and Implementation Center for assistance with SEM operation. This work was supported by a grant from the Erciyes University Scientific Research Office (FCD-2018-8242).
Author information
Authors and Affiliations
Contributions
S.D. performed all experiments as a first author. C.C. contributed to experiments I.O. conceived the original idea and designed the project. S.D., C.C. and I.O. mainly wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dadi, S., Celik, C. & Ocsoy, I. Gallic acid nanoflower immobilized membrane with peroxidase-like activity for m-cresol detection. Sci Rep 10, 16765 (2020). https://doi.org/10.1038/s41598-020-73778-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-73778-7
- Springer Nature Limited
This article is cited by
-
Antimicrobial and Anticancer Activity of Gallic Acid–Cu(II) Hybrid Nanoflowers and Gallic Acid–Zn(II) Hybrid Nanoflowers
Journal of Inorganic and Organometallic Polymers and Materials (2024)
-
Rational design of EDTA-incorporated nanoflowers as novel and effective endodontic disinfection against biofilms
Odontology (2024)
-
Ficin-copper hybrid nanoflowers with enhanced peroxidase-like activity for colorimetric detection of biothiols
Microchimica Acta (2023)
-
Characterization of green synthesized nanoflowers using corn silk extract obtained in different solvents and pH media and comparative study of the effects of morphologies on catalytic, antioxidant, and antimicrobial activities
Applied Nanoscience (2023)
-
BSA-Cu3(PO4)2 hybrid nanoflower—an efficient and low-cost nanoenzyme for decolorization of organic pollutants
Analytical and Bioanalytical Chemistry (2023)