Abstract
Lyme disease, caused by Borrelia burgdorferi, is an inflammatory multistage infection, consisting of localized, disseminated, and persistent disease stages, impacting several organ systems through poorly defined gene regulation mechanisms. The purpose of this study is to further characterize the spatiotemporal transcriptional regulation of B. burgdorferi during mammalian infection of borrelial oxidative stress regulator (bosR) and decorin binding protein (dbpBA) by utilizing bioluminescent B. burgdorferi reporter strains and in vivo imaging. Fluctuating borrelial load was also monitored and used for normalization to evaluate expression levels. bosR transcription is driven by two promoters, Pbb0648 and PbosR, and we focused on the native promoter. bosR expression is low relative to the robustly expressed dbpBA throughout infection. In distal tissues, bosR was the highest in the heart during in the first week whereas dbpBA was readily detectable at all time points with each tissue displaying a distinct expression pattern. This data suggests bosR may have a role in heart colonization and the induction of dbpBA indicates a RpoS independent transcriptional regulation occurring in the mammalian cycle of pathogenesis. These finding demonstrate that B. burgdorferi engages unknown genetic mechanisms to uniquely respond to mammalian tissue environments and/or changing host response over time.
Similar content being viewed by others
Introduction
Lyme disease, caused by Borrelia burgdorferi, is a complex vector-borne infection that causes a multistage inflammatory disease in numerous systems resulting in significant morbidity1,2,3,4. The multiple stages begin with a localized dermal colonization followed by a dissemination to distal immunoprotective niches, including the central nervous system (CNS), heart, and joints, that can result in the development of neuroborreliosis, Lyme carditis, and/or arthritis, respectively. A robust innate and adaptive immune response is elicited by B. burgdorferi that is unable to clear infection or prevent the progression of disease1. Antibiotic treatment effectively resolves the disease during early localized infection, but less so after dissemination and late Lyme disease. The Center for Disease Control (CDC) recently reported a substantial increase in the number of cases in the United States from approximately 30,000 to 300,000 per year due to under reporting and less than optimal diagnostic tools5. Lyme disease is a significant public health concern with limited treatment options to protect and maintain long term quality of life in humans.
Borrelia burgdorferi, a spirochetal bacterium, dynamically regulates gene expression to promote each stage of disease in the vector and mammalian host modulate disease and evade the immune response2,6,7,8. Changes in environmental cues, such as pH, temperature, O2, CO2, metals and osmotic stress, detected by B. burgdorferi result in differential presentation of lipoproteins on the outer membrane to promote adherence, invasion, and colonization9,10,11,12,13,14,15,16,17. Unknown host specific and tissue specific signals induce changes in gene expression that cannot be attributed to known environmental cues defined during in vitro cultivation18,19. Expression or repression of mammalian virulence determinants in a temporal and tissue specific manner is essential to progress through the stages of disease. For example, the ectopic constitutive expression of outer surface protein C, ospC, that is typically down regulated shortly after infection results in the clearance of the spirochetal pathogen days after inoculation20. The specific mechanisms required for borrelial dissemination, distal colonization, and long term infection of tissues are not well understood.
One well characterized mechanism important to establish localized infection is the BosR-Rrp2-RpoN-RpoS pathway that activates gene expression of mammalian virulence determinants in response to an infected tick acquiring a blood meal21,22,23,24,25,26,27,28,29,30. A complex comprised of the borrelial oxidative stress regulator (BosR), response regulator 2 (Rrp2), and sigma factor (RpoN) interacts with the promoter region of transcriptional activator, rpoS. Downstream in this pathway, RpoS activates expression of numerous lipoproteins including outer surface protein C (ospC) and decorin binding protein (dbpBA)31,32. BosR, a metalloregulatory transcriptional regulator, undergoes transcriptional and post-transcriptional regulation in response to environmental signals pH and CO2, respectively14,33. Murine infection requires bosR presumably due its activation of rpoS and indirectly the RpoS regulon, but bosR expression has not been previously evaluated in vivo22,24. OspC is required for establishing localized infection and phage display studies suggest it contributes to the colonization of the murine heart34,35,36,37. Our previous work with bioluminescent reporter B. burgdorferi strains to evaluate ospC transcript demonstrated unique levels and timing of expression in murine tissues38. We observed higher levels of expression in the heart during early infection and an increase of ospC in the bladder and joint during late infection. DbpA is also important for establishing a disseminated mammalian infection as it interacts with the extracellular matrices (ECM) through the binding to decorin and type I collagen39,40,41,42.
We hypothesize that gene regulation is necessary for B. burgdorferi to disseminate, colonize unique niches, and/or persist in distal sites during mammalian infection. To address this, we utilized bioluminescent in vivo reporter strains of B. burgdorferi to monitor in real time gene expression of bosR, dbpBA, and ospA during murine experimental infection and in specific tissues. This technology also allows for the evaluation of gene expression independent of variations in borrelial burden in distal colonized tissues. This study demonstrates that B. burgdorferi genetic regulation is increasingly complex and newly developed technology allows the dissection of more individualized events. Specifically, we show here that bosR is transcriptionally regulated during earlier stages of murine infection with the highest levels observed in the heart. Expression patterns of dbpBA are abundant throughout infection and distinct from other RpoS regulated genes, further indicated an independent regulatory mechanism.
Results
In vitro characterization of B. burgdorferi bioluminescent reporter strains for bosR, dbpBA, and ospA
To accomplish our goal of evaluating the gene expression patterns of specific genes during murine infection, we generated B. burgdorferi bioluminescent reporter strains for bosR, dbpBA, and negative control ospA similar to our previous studies38,39. It is important to note that bosR transcription is driven by a promoter immediately upstream (PbosR) evaluated in this study and the bb0648 promoter33. A borrelial codon optimized firefly luciferase (luc) developed in B. burgdorferi was utilized to link PbosR, Pdbp, and PospA in frame to drive luminescence production as a readout for gene expression43. Each reporter was cloned into multicopy borrelial shuttle vector pBBE22 that encodes nicotinamidase restoring mammalian infectivity in a lp25 deficient strain, resulting in pJH488, pJH481, and pJH486, for PbosR, Pdbp, and PospA, respectively (Table 1)44. This bioluminescent shuttle vector is maintained throughout mammalian infection without antibiotic selection due to the selective pressure to maintain the nicotinamidase gene and increases the sensitivity of detection38,39. The bioluminescent reporter shuttle vectors were transformed into lp25 deficient B. burgdorferi, ML23. The newly generated strains designated ML23 pJH488, ML23 pJH481, and ML23 pJH486 will be referred to as PbosR-luc, Pdbp-luc, and PospA-luc throughout this study for simplification (Table 1).
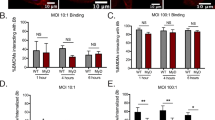
Our first step was to ensure the borrelial bioluminescent reporters were able to respond to environmental cues known to alter bosR, dbpBA, and ospA transcripts. Reporter strains were grown under pH 6.8 and 7.5 to mimic mammalian and tick conditions for in vitro luminescence assays and Western analysis (Fig. 1). Mammalian virulence determinants reporters PbosR-luc and Pdbp-luc are transcriptionally induced at pH 6.8 relative to pH 7.5, as expected (Fig. 1A, B)33,48. Western analysis demonstrated an increase of BosR and DbpA in PbosR-luc and Pdbp-luc strains, respectively, at pH 6.8 when compared to pH 7.5 (Fig. 1E). FlaB production was unchanged under the different pH conditions and used as an equivalent loading control for Western analysis. OspA an important lipoprotein for tick midgut colonization, therefore PospA-luc is a useful negative control during mammalian infection49,50. PospA-luc demonstrated elevated bioluminescence and OspA production at pH 7.5 as expected (Fig. 1C, E)48. The previously reported constitutive luminescent B. burgdorferi PflaB-luc was used as a negative control for a transcript not altered by environmental changes in vitro and a readout for bacterial load in vivo (Fig. 1D)38,39. Together, these results indicate the reporter strains appropriately respond to environmental cues to represent the transcriptional regulation of bosR, dbpBA, and ospA.
Bioluminescence response of in vitro cultivated B. burgdorferi reporter strains to pH. Borrelial strains (A) PbosR-luc, (B) Pdbp-luc, (C) PospA-luc, (D) PflaB-luc were grown in triplicate to mid-log phase and serially diluted from 106 to 10 cells. B. burgdorferi strains were treated with D-luciferin. Luminescence was measured for each sample after subtracting the background levels and averaged. The error bars in the graphs represent standard error. Statistical significance was determined by t-test (*p < 0.05 and **p < 0.01). (E) Cell lysates from PbosR-luc, Pdbp-luc, PospA-luc, and PflaB-luc were immunoblotted and probed with anti-sera to antigen indicated on the left. Constitutively produced borrelial FlaB was used as a control for cell equivalents between samples.
As with other bacterial pathogens, B. burgdorferi undergoes post-transcriptional regulation that is not addressed with this methodology, which is relevant for BosR that is transcriptionally and post-transcriptionally regulated14,33. PbosR-luc was grown in media equilibrated under 1% and 5% CO2 atmospheric conditions resulting in no difference in luminescent emission, but an increase in BosR production at elevated CO2 (Fig. 2). As in the previous experiment, PflaB-luc is a bioluminescent assay negative control for environmental changes and FlaB is a Western blot equivalent loading control. This study focused on a single step of genetic regulation of B. burgdorferi, specifically for bosR and dbpBA transcription, during murine infection.
Luminescence of B. burgdorferi PbosR-luc reporter strain to CO2. Triplicate borrelial strains, PflaB-luc and PbosR-luc, were grown in 1% or 5% CO2 to mid-log phase and samples were harvested for bioluminescence assay (A) or Western analysis (B). (A) Cells were serially diluted from 106 to 10 cells, treated with D-luciferin, and measured for bioluminescence. Samples were averaged and t-test analysis determined statistical significance. Error bars represent standard error. PflaB-luc and PbosR-luc demonstrate no difference in bioluminescence with changes in CO2 levels, as expected. (B) Samples were immunoblotted and probed with anti-sera to antigen indicated on the left. Constitutively produced borrelial FlaB was used as a control for cell equivalents between samples and are not impacted changes in cultivation conditions.
Temporal evaluation of B. burgdorferi reporter strains during murine infection
An in vivo bioluminescent reporter infectivity study was performed to characterize the dynamic regulation of bosR, dbpBA, and ospA during mammalian infection. B. burgdorferi PflaB-luc, PbosR-luc, Pdbp-luc, or PospA-luc were intradermally (ID) inoculated in Balb/c mice that were monitored by in vivo imaging for the emission of light. The constitutively expressed PflaB-luc is a control for fluctuations in borrelial burden over time and in different colonization sites that is used for normalization allowing for the analysis of changes in transcription only. Bioluminescent imaging, represented as photons/sec or radiance, are normalized for background utilizing an infected mouse not treated with D-luciferin in each group. As observed in previous bioluminescent studies with constitutive PflaB-luc a strong localized colonization develops at the site of inoculation followed by the dissemination of the bioluminescent signal to distal tissues and throughout the murine skin with bacterial load fluctuating throughout the 21 day time course (Figs. 3, 4A)38,39. At the brief 2 h time point bioluminescent levels are representative of B. burgdorferi response to in vitro microaerophilic growth conditions as indicated by the robust emission from PospA-luc infected mice (Fig. 3). ospA is one of the highest expressed genes during cultivation of B. burgdorferi48. PospA-luc bioluminescence is observed and measurable 2 h after ID injection, but quickly declines to background levels at all other time points (Figs. 3, 4A). The multicopy PospA-luc reporter is down regulated in the murine dermis within 24 h post-infection (data not shown) and does not return in the same manner as native ospA51,52. This demonstrates the in vivo bioluminescence B. burgdorferi reporter system can accurately represent bacterial burden and transcriptional changes, both positively and negatively, in the murine model.
Temporal expression B. burgdorferi reporter strains during experimental murine infection. The expression of bosR, dbpBA, and ospA during murine infection was evaluated over time. Bioluminescent B. burgdorferi PflaB-luc, PbosR-luc, Pdbp-luc, and PospA-luc infected Balb/c mice by intradermal injection with a 105 inoculum dose. PflaB-luc serves as a monitor of bacterial load. D-luciferin treatment, represented by + , was administered 1 h and 4, 7, 10, 14, and 21 days post-infection for in vivo imaging. A background control mouse (far left in each image and indicated by −) is included in each group that is not treated with D-luciferin. Exposures of 10 min was captured for images. Normalization of images was performed to remove all visible background bioluminescence from the no luciferin treated mouse across all strains and time points. All images from each time point and strain were normalized to the same radiance range of 8.89 × 103 to 5.89 × 105.
Quantitation of in vivo bioluminescence of B. burgdorferi reporter strains. (A) Exposures with counts range from 600 to 60,000 were used for quantitation of bioluminescence. One-way ANOVA showed significant difference in bioluminescence over time for PflaB-luc (p < 0.0001), PbosR-luc (p < 0.0001), Pdbp-luc (p < 0.0068), and PospA- (p < 0.0001). (B) PbosR-luc normalized for changes in borrelial load (PflaB-luc). Permutation analysis indicated no significant differences of PbosR-luc/PflaB-luc between time points. (C) Pdbp-luc normalized for changes in borrelial load with PflaB-luc. Permutation analysis was used to determine the significant difference between time points for each reporter strain. Pdbp-luc/ PflaB-luc on day 7 was significantly different relative to other time points with a p value range of 0.0016 to 0.0129. * represents p < 0.05 and ** designates p < 0.01.
We assessed the temporal transcriptional regulation of bosR during mammalian infection. Expression of bosR is low under in vitro microaerophilic condition as represented by the level of emission from PbosR-luc infected mice 2 h post infection (Fig. 3). PbosR-luc bioluminescence peaks with 4.3 × 105 photons/s (p/s) at 4 days post infection (dpi) followed by a 11.5-fold and 22-fold reduction at 7 and 21 dpi, respectively (Figs. 3, 4A). One-way ANOVA (p < 0.0001) indicates a significant difference in PbosR-luc bioluminescence during the course of a 21 day infection. Normalization of PbosR-luc for changes in borrelial burden, represented as PbosR-luc/PflaB-luc, indicates the highest expression of bosR is during the first 7 days of infection suggesting this transcriptional regulator is important for early stages of pathogenesis and establishing infection in the murine model (Fig. 4B). Permutation analyses of PbosR-luc/PflaB-luc did not show a significant difference when comparing time points likely due to low level signal and variability of bioluminescent emission between mice. The PbosR-luc in vivo expression pattern is similar to our previous study with PospC-luc, as one would expect, due to the role of BosR in the transcriptional activation of rpoS in complex with Rrp2 and RpoN22,23,24,25.
The RpoS regulation of dbpBA in response to environmental signals under in vitro cultivation conditions is well understood, while regulation of this important lipoprotein in physiologic environment of murine infection is less clear10,11,31,48. Expression of dbpBA during in vitro cultivation is substantially lower than flaB or ospA and is also observed in Balb/c mice intradermally inoculated for 2 h with Pdbp-luc (Figs. 1, 3, 4A). B. burgdorferi Pdbp-luc adapts to the murine host resulting in a dramatic increase in bioluminescence beginning at 4 dpi and continuing through 21 dpi (Figs. 3, 4A). One-way ANOVA analysis of Pdbp-luc photon/sec resulted in a p-value of 0.0068 indicating bioluminescence significantly changes over time. The bioluminescent emission from Pdbp-luc exceeds constitutive PflaB-luc at 7, 14, and 21 dpi indicating that B. burgdorferi robustly expresses this specific RpoS regulated lipoprotein during mammalian infection. Normalizing for bacterial load, as shown by Pdbp-luc/PflaB-luc, dbpBA expression increases 15.6 fold from 4 to 7 dpi that was followed by a 15.3 fold decline at 10 dpi (Fig. 4C). Bioluminescence increased fivefold from the low emission at day 10 to the second peak at 21 dpi. Overall the data suggests that high levels of dbpBA expression may contribute to borrelial pathogenesis during dissemination and maintain colonization of distal tissues (Fig. 4C). Pdbp-luc/PflaB-luc bioluminescence at 7 dpi is significantly different by permutation analysis in comparison to all time points with p values ranging from 0.0457 to 0.01032. The lack of significance between time points indicates the maintained robust expression of dbpBA. The bioluminescent emission of Pdbp-luc B. burgdorferi has a distinct in vivo temporal expression pattern compared to PbosR-luc or previously published PospC-luc, which peaked at 7 dpi followed by a decline in bioluminescence that rises again at day 21 (Figs. 3, 4A)38. The difference in PospC-luc and Pdbp-luc during murine infection is observed in the overall intensity and temporal production of bioluminescent emission when evaluating the whole mouse.
To verify mice were infected with equivalent numbers of B. burgdorferi PflaB-luc, PbosR-luc, Pdbp-luc, or PospA-luc harvested tissues underwent qualitative and quantitative evaluation (Fig. 5). At each day of imaging the inguinal lymph node, skin flank, ear, and the tibiotarsal joint were collected and transferred to complete BSK to monitor the outgrowth of viable B. burgdorferi (Fig. 5A). Tissues were qualitatively scored positive once motile borrelial cells were observed by dark field microscopy. All tissues have similar colonization of B. burgdorferi between all strains at each time point indicating groups of mice were inoculated with approximately the same number of cells (Fig. 5A). DNA was isolated from skin flank samples harvested at 4 and 14 dpi to determine the number of borrelial genomic copies (recA) per 106 mouse β-actin (Fig. 5B). One-way ANOVA and individual Mann–Whitney analysis indicated there was not a significant difference in the borrelial burden of PflaB-luc, PbosR-luc, Pdbp-luc, or PospA-luc infected skin flanks verifying mice were equivalently infected (Fig. 5B).
Bacterial burden of B. burgdorferi bioluminescent reporter strain infected mice. (A) Qualitative kinetics of infection with B. burgdorferi reporter strains in Balb/c mice. Upon completion of optical imaging for tissues from Balb/c mice infected with 105 PflaB-luc, PbosR-luc, Pdbp-luc, and PospA-luc, the animals were sacrificed and the skin flank, ear, inguinal lymph node and tibiotarsal joint were harvested on day 4, 7, 10, 14 and 21 for in vitro cultivation to monitor the outgrowth of B. burgdorferi. The x-axis indicates the strains and time points tested (in days). The y-axis displays the total percentage of culture positive tissues for a given time point comprised of the five tissues per strain. Groups of mice were equivalently infected with each reporter strain. (B) Bacterial burden equivalent between groups of mice infected with B. burgdorferi reporter strains. Flank skin samples of Balb/c mice infected with 105 PflaB-luc, PbosR-luc, Pdbp-luc, and PospA-luc were harvested on 4 and 14 dpi for qPCR analysis of borrelial genomes (recA) per copies of 106 β-actin. Error bars represent standard error. Statistical analysis using the Mann–Whitney test indicated a lack of significance between all strains at 4 and 14 dpi.
bosR expression in murine tissues
Transcriptional regulator bosR is regulated by environmental signal, temperature, pH, O2 and CO2, and is essential for murine infection9,14,33. B. burgdorferi differentially regulates gene expression in a tissue specific manner over the course of infection that is not replicated under environmental in vitro conditions2,18,38,53. The tissue specific transcriptional regulation of bosR during murine infection is unknown. Ex vivo imaging of individual murine tissues infected with bioluminescent B. burgdorferi allows for the direct spatiotemporal analysis of gene regulation while taking into account changes in borrelial burden. We harvested tissues, including skin flank, inguinal lymph node, heart, bladder, and tibiotarsal joints, from mice infected with bioluminescent B. burgdorferi, PflaB-luc, PbosR-luc, and Pdbp-luc reporters, to quantitate radiance emission at each time point beginning at 4 dpi (Figs. 6, S1). Similar to in vivo imaging, mice were treated with a double bolus of D-luciferin with the exception of 1 mouse that served as the background control for normalization.
Temporal and spatial expression of B. burgdorferi containing PflaB-luc, PbosR-luc, and Pdbp-luc in murine tissues. Individual tissues from Balb/c mice infected with from PflaB-luc, PbosR-luc, and Pdbp-luc B. burgdorferi were quantitatively evaluated for bioluminescence at 4, 7, 10, 14, and 21 dpi. PflaB-luc represents borrelial load. PbosR-luc and Pdbp-luc represents transcription of bosR and dbp, respectively. Tissues aligned under the (−) were not treated with D-luciferin serving as a background control and (+) designated tissues were harvested from mice treated with a double bolus of D-luciferin prior to sacrifice. Images of individual tissues are from 10 min exposures and normalized to the same radiance range (on the right) across all borrelial strains for each tissue. (A) Underside of flank skin (9.1e3–3.7e5 radiance), (B) inguinal lymph node (7.05e2–3.7e5 radiance), (C) heart (1.11e3-3.7e5 radiance), (D) BLADDER (6.9e2–3.7e5 radiance), (E) tibiotarsal joint (1.93e3–3.62e5 radiance).
PflaB-luc infected tissues were measured for the changes in bioluminescence at each site and time point to represent borrelial burden and serve as a normalization control in this study (Figs. 6, S1). Our previous work demonstrated a strong correlation between PflaB-luc bioluminescence emission and borrelial load39. PflaB-luc infected skin, heart, and joint showed statistically significant differences over time with a p values of < 0.0001, 0.0168, and 0.0007, respectively, by one-way ANOVA (Fig. S1). The inguinal lymph node and bladder did not significantly fluctuate over time and were infected at consistent levels throughout infection. Overall, each examined tissue displayed a distinct PflaB-luc bioluminescence signal indicating the unique microenvironment of each site alters the number of B. burgdorferi at a given time. The radiance from PflaB-luc infected tissues were used to normalize bioluminescence from PbosR-luc infected mice to account for differing borrelial burden (Fig. 7).
Tissue specific regulation of bosR and dbpBA expression independent of fluctuation in B. burgdorferi burden. To account for changes in bacterial load during the course of infection PbosR-luc infected tissues were normalize to PflaB-luc resulting in the ratio PbosR/PflaB. (A) Underside of skin flank, (B) inguinal lymph node, (C) heart, (D) bladder, (E) tibiotarsal joint. Permutation analyses was perform to randomly compare all possible ratios and determine statistical significance with * representing p < 0.05, **p < 0.01, and ***p < 0.001.
We evaluated the spatiotemporal expression of transcriptional regulator bosR during infection in PbosR-luc inoculated mice (Fig. 6). Radiance (p/sec/cm2/sr) was measured from tissues of four D-luciferin treated PbosR-luc infected mice and normalized for background to one untreated infected mouse (Figs. 6, S1). Over the course of infection bioluminescent emission significantly changed when analyzed by one-way ANOVA in the skin flank, heart, and bladder with p-values of 0.0028, 0.00035, and 0.0155, respectively. Bioluminescent emission in the lymph node was low and highly variable during murine infection indicating bosR was not steadily induced or necessary in this particular tissue in a manner previously observed with PospC-luc (Figs. 6, S1B)38. The joint was consistently infected as observed in IVIS images, thus did not significantly differ when comparing time points (Figs. 6, S1E). The ratio PbosR-luc/PflaB-luc represents bosR expression normalized for changes in borrelial burden (Fig. 7). The temporal expression of bosR is very similar to ospC in several tissues39. Specifically, PbosR-luc/PflaB-luc have higher ratios at earlier time points in the skin flank and heart (Fig. 7A, C). Expression in the bladder steadily declines over time (Fig. 7D). In the joint, ratios peak at 4 and 21 dpi as observed with PospC-luc/PflaB-luc (Fig. 7E). Permutation analysis of PbosR-luc/PflaB-luc ratios was performed and only the heart was significantly different between 7 versus 10 dpi, 10 versus 14 dpi, and 10 versus 21 dpi with p-values of 0.0123, 0.0111, and 0.0111, respectively (Fig. 7C). Together, this data indicates that bosR has moderate transcriptional regulation during murine infection over time in a tissue specific manner. It is likely that post-transcriptional regulation of bosR has a greater impact on the RpoS pathway and murine pathogenesis.
Data from imaging of PbosR-luc infected tissues was validated by utilizing qRT-PCR to ensure that bioluminescence accurately represents native bosR transcript levels. Total copies of bosR were quantitated from skin and heart harvested at 10 and 21 dpi (Fig. S2). Radiance from each PbosR-luc tissue from ex vivo imaging samples were correlated with total bosR transcript resulting in a strong correlation of 0.8245 and 0.9521 for skin and heart, respectively (Fig. 8A, B). This indicates the bioluminescent emission from PbosR-luc infected tissues is representative of bosR transcription during infection.
Correlation of reporter driven bioluminescence and native transcript for bosR and dbpBA. To validate the bioluminescent reporters encoded on a multicopy shuttle vector accurately reflects native transcription a correlation of radiance and copies of the gene of interest within a specific tissue was performed. Total transcript copies of bosR from the skin flank (A) and heart (B) from 10 and 21 dpi was correlated with the representative radiance value. Additionally, dbpBA copies from the skin flank (C) and heart (D) from 10 and 21 dpi was correlated with appropriate radiance.
Unique expression patterns of dbpBA throughout murine infection in all assessed tissues
The expression of dbpBA is important to establish a robust colonization during early localized murine infection39,40,54,55,56. Robust expression of dbpBA during murine infection suggests a role in dissemination and colonization of specific tissues52,53. Our bioluminescent Pdbp-luc borrelial strain was used to quantitate gene expression relative to borrelial burden in numerous tissues with the progression of infection (Figs. 6, 7, S1). Bioluminescent emission was readily observed from all tissues at each time points with the exception of joints 4 dpi and hearts at 4 and 7 dpi (Fig. 6). Radiance quantitation clearly demonstrated distinct expression patterns in each tissue that was significantly different by one-way ANOVA for all tissues over time (Fig. S1). Specifically, Pdbp-luc in the skin flank peaks on 4 dpi (5.3 × 104 p/s/cm2/sr) followed by a dramatic and maintained tenfold reduction at 10dpi out to 21dpi as observed during in vivo imaging (Figs. 6, S1A). The bioluminescent radiance in the lymph node emits steady state levels of dbpBA that is substantially higher than PbosR-luc (Fig. S1B). Pdbp-luc bioluminescence in the heart increases until reaching a peaking at 1.26 × 104 p/s/cm2/sr on 14 dpi (Fig. S1C). Bladder bioluminescence peaks early and rapidly declines similar to Pdbp-luc infected skin (Fig. S1D). Joint bioluminescence steadily increases until the final 21 dpi time point with an overall 602-fold increase relative to 4 dpi (Fig. S1E).
Normalization of Pdbp-luc radiance values with PflaB-luc reveals unique changes in expression not observed by evaluating Pdbp-luc or dbpBA alone (Fig. 7). Specifically, skin Pdbp-luc/PflaB-luc indicates a higher level of dbpBA expression during borrelial colonization at 7 dpi that is significantly different than all compared time points (Fig. 7A). Bioluminescence levels of Pdbp-luc are higher than PflaB-luc at 7, 14, and 21 dpi. Permutation analyses of lymph node Pdbp-luc/PflaB-luc bioluminescence found no significant differences between time points indicating a steady state expression of dbpBA in this tissue unlike the other distal sites evaluated (Fig. 7B). Infected hearts and bladders normalized for borrelial loads demonstrated peak Pdbp-luc bioluminescence at 7 dpi and were significantly different from all time points (Fig. 7C, D). Tibiotarsal joints displayed the most distinct bioluminescence profile increasing 31-fold when peaking at 21 dpi (Fig. 7E). As was performed with PbosR-luc infected tissues, qRT-PCR was used to correlate native dbpBA transcript levels with bioluminescence from the multicopy reporter (Figs. 8, S2). Correlation values of 0.9010 and 0.8845 for the heart and joint, respectively, were observed at 10 and 21 dpi (Fig. 8C, 8D). This data indicates that bioluminescence quantitated from Pdbp-luc encoded on a shuttle vector accurately represents dbpBA expression. Overall, B. burgdorferi dbpBA is strongly expressed in all tissues at most time points further supporting an important role for this operon during murine infection27,52,53. Together, this data demonstrates dbpBA regulatory patterns are distinct from expected RpoS driven regulation that was observed previously for PospC-luc38.
Discussion
Lyme disease progresses in multiple stages from localized infection, through dissemination, and establishes a long-term infection of distal tissues1,2,3. B. burgdorferi is a highly invasive bacterial pathogen that responds to distinct environmental pressures as it progresses through stages of mammalian infection2,57. Different tissues in the mammalian host presents an unique combination of environmental signals and ligands for interactions requiring B. burgdorferi to adapt to each site to colonize and maintain an infection. The borrelial gene regulation that occurs during the different stages of disease and tissues is not fully characterized. Further understanding disseminated or late Lyme disease will provide an opportunity to develop therapeutics beyond the window for effective antibiotic treatment. To date, serodiagnostics tests for Lyme diseases cannot identify early borrelial infections and a human vaccine is not available58. Therefore, patients are often diagnosed after dissemination has occurred and symptoms causing severe morbidity have arisen.
To further elucidate the complex spatiotemporal gene regulation of B. burgdorferi, we developed bioluminescent borrelial reporter strains allowing us to utilize highly sensitive in vivo imaging to quantitatively evaluate changes in bacterial load and gene expression throughout murine infection and individual tissues38,39. In this study, we generated bioluminescent reporter strains for genes encoding a borrelial transcriptional regulator, bosR, and adhesive lipoproteins, dbpBA, that are known to be important for mammalian infection, in part, due to the interplay with the transcriptional activator RpoS22,23,24,25,31,48. The absence of bosR results in the loss of rpoS activation and the reduction of RpoS regulated genes, including ospC and dbpBA22,23,24,25. In vivo imaging of PbosR-luc infected mice demonstrated low level expression throughout the 21 day infection relative to constitutively expressed (PflaB-luc) or Pdbp-luc infected mice. This was a similar expression pattern to that previously observed with PospC-luc B. burgdorferi with the exception of notable increase of ospC expression at 21 dpi38. Individual tissues elicited distinct levels of bosR expression with the heart emitting the highest level of bioluminescence, specifically at 4 and 7 dpi. The lowest bioluminescence from PbosR-luc infected tissues were from the inguinal lymph nodes and the bladder. An intermediate level of bosR expression was induced in the tibiotarsal joint and skin flanking the inoculation site with both tissues having the highest expression at 4dpi. The tibiotarsal joint also has substantial bosR expression at 21 dpi. Together, this data suggest transcriptional regulation of bosR occurs during murine infection largely during the early stages of colonizing an individual tissue. While bosR is essential for murine infection, low level expression is sufficient to support infection and is not required in all distal tissues. These findings are not in complete agreement with Ouyang et al. that molecularly quantitated bosR expression during the tick and murine cycle of infection53. Their study found higher levels of bosR expression at different time points in some of the individual tissues evaluated in this report. Specifically, bosR expression in the skin was abundant and increasing at 7, 14, and 21 dpi. It is not clear the source of the skin sample relative to tick attachment and could contribute to different expression patterns. They also found a substantial increase in bosR transcript levels at 7, 14, and 21 dpi in the bladder where in our study we found a relatively steady state low level of expression in this tissue. Differences in findings may be due to routes of infection, sensitivity of detection, and/or distinct methodologies. In vivo imaging allows for less manipulation of the tissues during ex vivo imaging with a high level of sensitivity for viable B. burgdorferi. In regards to the Ouyang et al. study, pooling of tissue RNA samples may have also been a contributing factor to distinct findings in regards to bosR expression53.
BosR, BB0647, is a transcriptional regulator that was initially identified as a Fur-like metalloregulatory transcriptional regulator to combat the oxidative stress response B. burgdorferi faces during tick blood meal and mammalian infection59,60,61,62. More recent studies suggest BosR is a potential global transcriptional regulator for the adaptation to mammalian infection and nutritional requirements14,63. As the second member of a three gene operon, bosR transcription is driven from two promoters, Pbb0648 and PbosR, but little is known about the role of the individual promoters on bosR expression33. Here, we have utilized PbosR in our bioluminescent reporter. The transcriptional regulation of bosR is similar to other borrelial genes that are responsive to pH, temperature, and growth phase33,64. Expression of bosR is also induced during nutrient stress in a RelBbu, responsible for the synthesis and hydrolysis of (p)ppGpp, dependent manner65. BadR, OppA4, and CsrA have proposed roles in bosR transcriptional regulation64,66,67,68. BadR and OppA4 repress bosR expression and the presence of CsrA is responsible for an increase in bosR transcription. Ouyang et al. evaluated potential autoregulation of BosR demonstrating minimal reduction in transcription in the absence of bosR33. Together, this suggests the transcriptional regulation of bosR occurs under a variety of conditions. Our findings in this study indicate that bosR transcriptional regulation may play a minor role influencing the outcome of borrelial pathogenesis.
It is possible that other levels of regulation or the metal dependent active state of BosR are more important to the regulation of B. burgdorferi during mammalian infection. We previously showed that post-transcriptional regulation of BosR occurs dependent on the concentration of CO214. Metals have an important role in regulation and active state of BosR. Manganese, zinc, and copper have been shown to post-transcriptionally regulate BosR production15,17. Specifically, manganese represses and zinc promotes BosR synthesis15. The metal binding ability of BosR is a point of debate in the field. Wang et al. found BosR is able to bind zinc, iron, and copper. In their study, copper and zinc had an inhibitory effect on DNA binding by BosR. A recent study focused on the metal binding ability of the two conserved CXXC motifs within BosR and found these sites were important for zinc binding, but not copper or iron69. Mutagenesis of the CXXC motifs reduced the production of RpoS and OspC. A conserved arginine at residue 39 in BosR is important for protein function in regards to combatting oxidative stress, DNA binding, OspC production, and murine infectivity60,70. The essential requirement for BosR during mammalian infection may be contributed to the active state of the functional protein through metal binding, oxidative state, and/or DNA binding abilities. The specific mechanisms that post-transcriptionally regulate bosR or how they influence pathogenesis in the murine host are not fully characterized. More broadly, the understanding of post-transcriptional regulation and the influence of sRNA encoded with the borrelial genome has only begun to be identified and pursued71,72,73.
We also evaluated the spatiotemporal expression of BosR-Rrp2-RpoN-RpoS regulated genes, dbpBA, utilizing in vivo bioluminescence reporter B. burgdorferi. During murine infection PdbpBA-luc emits abundant bioluminescence throughout the 21 day infection (Figs. 3, 4). Two peaks are observed during early dissemination (7 dpi) and late infection (21 dpi). This is distinct from two other members of the BosR-Rrp2-RpoN-RpoS pathway, bosR and ospC. Specifically, the highest Pdbp-luc bioluminescence is observed in the skin flank and heart during dissemination (Figs. 6, 7). The dbpBA expression is observed in the inguinal lymph node and tibiotarsal joint at a high level for each time point with the highest at late infection. Expression of ospC is minimally induced or absent in the inguinal lymph node38. Overall, our study demonstrates the overall induction of dbpBA expression throughout murine infection in all evaluated tissues. It is also clear that dbpBA undergoes RpoS independent regulation during mammalian infection by an unknown mechanism.
B. burgdorferi ability to interact with decorin is important for murine infection41. Mice lacking decorin are resistant to borrelial infection74. Alternatively, B. burgdorferi lacking dbpBA has an attenuated or a non-infectious phenotype in C3H and Balb/c mice, respectively39,40. This correlates with induced expression of dbpBA in response to mammalian environmental signals, including low pH, elevated temperature and CO211,14,48,75. During tick transmission of B. burgdorferi, the expression of dbpBA is delayed relative to ospC and is not observed prior to dermal colonization55. Abundant expression of dbpBA throughout murine infection has been observed in other studies52,53. The differences in dbpBA expression during murine infection and DMC incubation may be due to B. burgdorferi ability to interact with host tissues. Specifically, Hodzic et al. observed expression of dbpBA in skin, heart, and tibiotarsal joint of C3H and C3H-scid mice over the course of an 8 week infection52. The changes in transcript levels reported did not account for fluctuations in bacterial load through the course of infection or between the mouse strains. Higher levels of dbpBA expression in C3H-scid were likely due to higher borrelial burden. Additional studies evaluating dbpBA expression in SCID mice suggest the humoral response does not influence regulation of these genes in B. burgdorferi76. Ouyang et al. intradermally infected C3H mice and harvested skin, heart, and bladders for quantitative RT-PCR analysis of borrelial transcriptional response during murine infection normalized to flaB transcripts53. In this study, dbpBA was expressed at high levels in the bladder at all time points out to 50 dpi, whereas skin and heart had much lower or not detectable expression of the transcript. This is distinct from our study where Pdbp-luc emits quantitative bioluminescence at all time points. Overall, Ouyang et al. showed lower levels of detection of dbpBA at most time points in comparison to our bioluminescent in vivo reporter strain that may be attributed to the methodology requiring more manipulation to isolate RNA and reverse transcriptase-dependent dectection33. This suggests that ex vivo imaging of tissues infected with bioluminescent borrelial strains obtains a higher level of sensitivity relative to molecular methods. Our method also has the advantage of evaluating individual tissues and detecting the natural variability that is innate to murine experimental infection.
While our study is insightful to the understanding of borrelial transcriptional regulation during murine infection it is not without limitations. The bioluminescent reporter strains cannot address post-transcriptional or translational regulation that is known to be utilized by B. burgdorferi. Nor does it determine the presentation of DbpA on the surface of the bacterial cell or the active state of BosR that requires metal binding and response to the oxidative state of the environment. These considerations require further investigation and methodologies that can be applied during murine infection. A second drawback to this study is the inability to perform tick transmission murine infections because the bioluminescence strains were generated in the ML23 background lacking lp25 that is required for tick and mammalian infection. The presence of pncA on the luciferase shuttle vector restores the ability to infect mice and serves as an in vivo selective pressure assuring that bioluminescence will not be lost over time39,44. Ideally, a single borrelial strain can be developed with two independent bioluminescent and/or biofluorescent alleles for evaluating bacterial load and expression of a gene of interest in a background strain that can infect ticks and mice while maintaining emission throughout a long term infection.
Future investigation is needed to understand the B. burgdorferi genetic regulation necessary for successful dissemination, colonization of distal tissues, and maintenance of long term infection. Specifically, the identification of host-specific environmental signals, the immune response, and host–pathogen interactions that may influence the expression of borrelial genes. Our study, and others, shows that dbpBA is distinctly regulated from ospC suggesting an unique regulatory mechanism from RpoS occurs during mammalian infection that has not been identified under in vitro cultivation conditions. Regulation of bosR is not limited to transcript levels, but includes post-transcription regulation in response to CO2 by a yet to be identified mechanism14. Recent identification of numerous sRNA encoded in the B. burgdorferi genome along with 5′ and 3′ untranslated regions (UTR) are possible candidates to explain the post-transcriptional regulation of bosR73. We largely do not understand the post-transcriptional regulation that is utilized by B. burgdorferi during mammalian infection. By extension, it is highly likely that similar, but distinct regulatory mechanisms are utilized during the tick stage of the pathogenic life cycle.
In this study, we have shown that B. burgdorferi uniquely regulates genes dependent upon the tissue that presents unique environmental signals and interactions. This genetic regulatory response varies over time in a manner that may be attributed to changes in the immune response. Specifically, transcriptional regulation of bosR and dbpBA occurs during murine infection distinctly in various tissues for two genes in the same regulatory pathway. Our study specifically focused on the transcriptional regulation from one of two promoters that drives bosR transcription and does not address post-transcriptional regulation of bosR. This is the first step in unraveling the complex regulation of bosR. The transcriptional regulation of dbpBA is likely independent of RpoS during a portion of murine infection. It is unclear when RpoS regulation of dbpBA is required. It is likely that other as yet unidentified mammalian specific regulatory mechanism are important for borrelial pathogenesis.
Materials and methods
Bacterial strains and culture conditions
Low-passage B. burgdorferi B31 were routinely cultured in BSK-II medium supplemented with 6% normal rabbit serum (Pel-Freez, Biologicals, Rogers, AR) (Table 1)77. Spirochetes were enumerated using dark-field microscopy. B. burgdorferi were grown to mid-exponential phase at 37 °C with in BSK-II medium adjusted to pH 6.8 or pH 7.5 at 1% CO2 or pH 7.5 with 1% CO2 or 5% CO2. All cultures were inoculated at a density of 105 cells/ml and harvested for luminescence and protein samples when cultures reached log phase. When appropriate 300 μg/ml kanamycin was added to the BSK-II medium. Escherichia coli were grown in Luria broth (LB) at 37 °C supplemented with the appropriate antibiotics, spectinomycin (100 μg/ml) or kanamycin (50 μg/ml).
Generated constructs and modification of B. burgdorferi
The promoter regions of genes of interest were amplified using forward and reverse primers as listed in Table 2 then cloned into pCR8/GW/TOPO (Invitrogen). Promoter regions for bosR, dbpBA, and ospA were amplified 393 bp, 362 bp, and 431 bp, respectively, upstream of the ATG. A construct encoding Bbluc lacking a promoter was generated with SalI, XhoI, ClaI, and NdeI restriction sites for promoter cloning in pCR8/GW/TOPO, designated pJH434. To obtain the final construct, the promoter linked to Bbluc was cloned into pBBE2244. The final construct was screened by restriction digest, luminescence, and confirmed by sequence analysis. This cloning design resulted in borrelial luciferase reporters for PbosR-luc (pJH481), Pdbp-luc (pJH488), and PospA-luc (pJH486). The transformations of B. burgdorferi ML23 with bioluminescent reporter shuttle vectors were carried out as described previously78. Transformed cultures were plated by limiting dilution in the presence of antibiotic selection. Positive transformants for were confirmed by bioluminescence assays, PCR screening for reporter plasmid and borrelial plasmid content45. For clarity the resulting strains ML23 pJH481, ML23 pJH488, and ML23 pJH482 are designated PbosR-luc, Pdbp-luc, and PospA-luc, respectively, (Table 1). Previously generated ML23 pBBE22luc is also designated PflaB-luc39.
SDS-PAGE and immunoblot analysis
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot analysis were performed as previously described9. Briefly, cell lysates were subjected to SDS-PAGE and transferred to a PVDF membrane for Western analysis. Proteins were detected using mouse monoclonal antisera to B. burgdorferi flagellum (Affinity BioReagents), rabbit anti-DbpA, rabbit anti-BosR, mouse anti-OspA, respectively. The following secondary antibodies were used for additional amplification: rabbit anti-mouse IgG conjugated with horseradish peroxidase (HRP), and anti-rabbit HRP.
In vitro bioluminescence assays
Three independent cultures of PflaB-luc, PbosR-luc, Pdbp-luc and PospA-luc were grown to mid-log phase and concentrated to 107 cells/ml. Cells were serially diluted from 106 to 10 cells and 100 μl of each appropriate dilution were distributed in a white flat-bottom microtiter 96 well-plate. Each sample for each strain was treated with a fresh 2 mM D-luciferin (GoldBio) diluted in PBS and luminescence was measured using 2104 EnVision Multilabel Reader (Perkin Elmer). The values of three independent cultures were averaged and the standard error was calculated.
In vivo and ex vivo bioluminescence infectivity studies and quantitation
Mice were cared for under standard ABSL-2 parameters during housing and IVIS imaging under the supervision of a Texas A & M University veterinarian. General health and wellness of the mice were monitored daily. No unexpected illnesses, distress, or deaths occurred during the time course of the infection. During imaging mice were given isoflurane as an anesthetic. Per the guidelines of the American Veterinary Medical Association (AVMA) and as approved by the Texas A & M University Institutional Animal Care and Use Committee (IACUC) mice were euthanized at the predetermined time points.
Bioluminescent imaging of mice and tissues harvest was performed as previously described38,39. Groups of 5 female 6–8 week old Balb/c mice (Charles Rivers) were infected by intraperitoneal injection (IP) with 105 B. burgdorferi PflaB-luc, PbosR-luc, Pdbp-luc, or PospA-luc. 5 mg D-luciferin dissolved in PBS was delivered to 4 of 5 mice by intraperitoneal (IP) injection. After 10 min mice were imaged at 2 h and 1, 4, 7, 10, 14 and 21 days post-infection (dpi) using the Perkin Elmer IVIS Spectrum live imaging system. One mouse not treated with D-luciferin serves as a background control for each group and time point. Exposures between 600–60,000 counts were used to quantitate bioluminescence, photons/sec (p/s) or radiance (p/s/cm2/sr) utilizing the regions of interest (ROI) tool from the Perkin Elmer Living Image Software. Bioluminescence from D-luciferin treated mice were normalized by subtracting background bioluminescence from the untreated mouse. Normalized bioluminescence values were averaged and standard error calculated. All images are a 10 min exposure and normalized for background across all borrelial strains and time points as represented by the colorimetric radiance scale.
Bioluminescence of individual infected tissues were measured during ex vivo imaging after mice were treated with 10 mg of D-luciferin 10 min prior to sacrifice and harvesting of skin, inguinal lymph node, heart, bladder, and tibiotarsal joint. Harvested tissues were transferred to 4 mM D-luciferin and 2 mM ATP solution and soaked for 3 min to avoid dehydration during imaging. As with in vivo imaging, one mouse was not treated with D-luciferin and tissues were transferred to 1X PBS prior to imaging. Bioluminescence of tissues was performed as described above for in vivo imaging. Tissues were flash frozen in liquid nitrogen and stored at -80 °C until isolation of total RNA for qRT-PCR. Skin samples were also collected to determine bacterial load.
Quantitative PCR and RT-PCR analysis
As previously performed, DNA or RNA was extracted from infected tissues to quantitate borrelial burden or native transcript levels38. B. burgdorferi infected skin flanks were processed with the Roche High Pure PCR Template Preparation kit to isolate genomic DNA. The Applied Biosystems ABI Step One was used to quantitate bacterial equivalents from infected mouse tissues. Borrelial genomic equivalents were quantitated in numbers of borrelial recA per 105 mouse β-actin as previously described from 100 ng of genomic DNA (Table 2)40. A Ct standard curve of calculated amounts of pβ-actin and precA standard plasmids to determine quantities from the Ct values of the experimental samples. Samples were measured in technical triplicate.
RNA was extracted from infected tissue by phase separation with Trizol per manufacture instructions (ThermoFisher) as previously performed38. Briefly, tissues were homogenized in Trizol, then treated with 200 μl of chloroform per ml of Trizol. Centrifugation of samples allowed phase separation and the upper aqueous phase containing RNA was precipitated. Total RNA (≤ 30 μg) was treated with 5 units Roche recombinant DNase to remove contaminating DNA from the sample. cDNA was generated from 3 μg of DNase treated RNA using Superscript III and random hexamers in a 20 μl reaction per manufacturer instructions (ThermoFisher). Transcript levels for each target, bosR and dbpA, was evaluated by qPCR with 3 μl of cDNA, 360 nM primers, and PowerUp Sybr (Thermofisher) (Table 2). A standard curve was utilized to determine total transcript levels of each tissue evaluated.
Statistical analyses
Statistical analysis of in vitro luminescence assays, in vivo bioluminescence (p/s), and ex vivo bioluminescence (p/s/cm2/sr) was performed using GraphPad Prism (GraphPad Software, Inc, La Jolla, CA). Significance was determined by p values equal to or less than 0.05. Bioluminescence temporal differences of individual strains was analyzed by one-way analysis of variance (ANOVA). Mann–Whitney one-tail test compared differences between bacterial strains at a single time point. Correlation was performed between quantitated bioluminescence and native transcript levels. To determine the significant differences over time of PbosR-luc/PflaB-luc and Pdbp-luc/PflaB-luc radiance ratios we used permutation tests to compute sampling distributions as previously described38. Permutation tests were performed using R, a freely available language and environment for statistical computing and graphics (ver. 3.2.3; https://cran.r-project.org/).
Ethics statement
In accordance with National Institute of Health (NIH) Guide for Care and Use of Laboratory Animals and Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) guidelines animal experiments were performed as described above. The Texas A & M University Institutional Animal Care and Use Committee (IACUC) approved all animal protocols and procedures, including but not limited to method for euthanasian.
References
Steere, A. C. et al. Lyme borreliosis. Nat. Rev. Dis. Primer 2, 16090 (2016).
Radolf, J. D., Caimano, M. J., Stevenson, B. & Hu, L. T. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat. Rev. Microbiol. 10, 87–99 (2012).
Stanek, G., Wormser, G. P., Gray, J. & Strle, F. Lyme borreliosis. Lancet 379, 461–473 (2012).
Steere, A. C., Coburn, J. & Glickstein, L. The emergence of Lyme disease. J. Clin. Investig 113, 1093–1101 (2004).
Mead, P. S. Epidemiology of Lyme disease. Infect. Dis. Clin. N. Am. 29, 187–210 (2015).
Caimano, M. J., Drecktrah, D., Kung, F. & Samuels, D. S. Interaction of the Lyme disease spirochete with its tick vector. Cell. Microbiol. 18, 919–927 (2016).
Samuels, D. S. Gene regulation in Borrelia burgdorferi. Annu. Rev. Microbiol. 65, 479–499 (2011).
Groshong, A. M. & Blevins, J. S. Insights into the biology of Borrelia burgdorferi gained through the application of molecular genetics. Adv. Appl. Microbiol. 86, 41–143 (2014).
Seshu, J., Boylan, J. A., Gherardini, F. C. & Skare, J. T. Dissolved oxygen levels alter gene expression and antigen profiles in Borrelia burgdorferi. Infect. Immun. 72, 1580–1586 (2004).
Carroll, J. A., Garon, C. F. & Schwan, T. G. Effects of environmental pH on membrane proteins in Borrelia burgdorferi. Infect. Immun. 67, 3181–3187 (1999).
Carroll, J. A., Cordova, R. M. & Garon, C. F. Identification of 11 pH-regulated genes in Borrelia burgdorferi localizing to linear plasmids. Infect. Immun. 68, 6677–6684 (2000).
Brooks, C. S., Hefty, P. S., Jolliff, S. E. & Akins, D. R. Global analysis of Borrelia burgdorferi genes regulated by mammalian host-specific signals. Infect. Immun. 71, 3371–3383 (2003).
Stevenson, B., Schwan, T. G. & Rosa, P. A. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect. Immun. 63, 4535–4539 (1995).
Hyde, J. A., Trzeciakowski, J. P. & Skare, J. T. Borrelia burgdorferi alters its gene expression and antigenic profile in response to CO2 levels. J. Bacteriol. 189, 437–445 (2007).
Troxell, B. et al. Manganese and zinc regulate virulence determinants in Borrelia burgdorferi. Infect. Immun. 81, 2743–2752 (2013).
Bontemps-Gallo, S., Lawrence, K. & Gherardini, F. C. Two different virulence-related regulatory pathways in Borrelia burgdorferi are directly affected by osmotic fluxes in the blood meal of feeding Ixodes ticks. PLoS Pathog. 12, e1005791 (2016).
Wang, P. et al. BosR is a novel fur family member responsive to copper and regulating copper homeostasis in Borrelia burgdorferi. J. Bacteriol. 199(16), e00276-17 (2017).
Akins, D. R., Bourell, K. W., Caimano, M. J., Norgard, M. V. & Radolf, J. D. A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J. Clin. Investig. 101, 2240–2250 (1998).
Iyer, R. et al. Stage-specific global alterations in the transcriptomes of Lyme disease spirochetes during tick feeding and following mammalian host adaptation. Mol. Microbiol. 95, 509–538 (2015).
Xu, Q., Seemanapalli, S. V., McShan, K. & Liang, F. T. Constitutive expression of outer surface protein C diminishes the ability of Borrelia burgdorferi to evade specific humoral immunity. Infect. Immun. 74, 5177–5184 (2006).
Yang, X. F., Alani, S. M. & Norgard, M. V. The response regulator Rrp2 is essential for the expression of major membrane lipoproteins in Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 100, 11001–11006 (2003).
Hyde, J. A., Shaw, D. K., Smith, R. III., Trzeciakowski, J. P. & Skare, J. T. The BosR regulatory protein of Borrelia burgdorferi interfaces with the RpoS regulatory pathway and modulates both the oxidative stress response and pathogenic properties of the Lyme disease spirochete. Mol. Microbiol. 74, 1344–1355 (2009).
Hyde, J. A., Shaw, D. K., Smith, R., Trzeciakowski, J. P. & Skare, J. T. Characterization of a conditional bosR mutant in Borrelia burgdorferi. Infect. Immun. 78, 265–274 (2010).
Ouyang, Z. et al. BosR (BB0647) governs virulence expression in Borrelia burgdorferi. Mol. Microbiol. 74, 1331–1343 (2009).
Ouyang, Z., Deka, R. K. & Norgard, M. V. BosR (BB0647) controls the RpoN-RpoS regulatory pathway and virulence expression in Borrelia burgdorferi by a novel DNA-binding mechanism. PLoS Pathog. 7, e1001272 (2011).
Blevins, J. S. et al. Rrp2, a sigma54-dependent transcriptional activator of Borrelia burgdorferi, activates rpoS in an enhancer-independent manner. J. Bacteriol. 191, 2902–2905 (2009).
Caimano, M. J. et al. Analysis of the RpoS regulon in Borrelia burgdorferi in response to mammalian host signals provides insight into RpoS function during the enzootic cycle. Mol. Microbiol. 65, 1193–1217 (2007).
Burtnick, M. N. et al. Insights into the complex regulation of rpoS in Borrelia burgdorferi. Mol. Microbiol. 65, 277–293 (2007).
Ouyang, Z., Blevins, J. S. & Norgard, M. V. Transcriptional interplay among the regulators Rrp2, RpoN and RpoS in Borrelia burgdorferi. Microbiology 154, 2641–2658 (2008).
Smith, A. H., Blevins, J. S., Bachlani, G. N., Yang, X. F. & Norgard, M. V. Evidence that RpoS (sigmaS) in Borrelia burgdorferi is controlled directly by RpoN (sigma54/sigmaN). J. Bacteriol. 189, 2139–2144 (2007).
Hubner, A. et al. Expression of Borrelia burgdorferi OspC and DbpA is controlled by a RpoN-RpoS regulatory pathway. Proc. Natl. Acad. Sci. USA 98, 12724–12729 (2001).
Yang, X. F. et al. Analysis of the ospC regulatory element controlled by the RpoN-RpoS regulatory pathway in Borrelia burgdorferi. J. Bacteriol. 187, 4822–4829 (2005).
Ouyang, Z., Zhou, J. & Norgard, M. V. Evidence that BosR (BB0647) is a positive autoregulator in Borrelia burgdorferi. Infect. Immun. 84, 2566–2574 (2016).
Antonara, S., Chafel, R. M., LaFrance, M. & Coburn, J. Borrelia burgdorferi adhesins identified using in vivo phage display. Mol. Microbiol. 66, 262–276 (2007).
Grimm, D. et al. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc. Natl. Acad. Sci. USA 101, 3142–3147 (2004).
Tilly, K., Bestor, A., Jewett, M. W. & Rosa, P. Rapid clearance of Lyme disease spirochetes lacking OspC from skin. Infect. Immun. 75, 1517–1519 (2007).
Tilly, K. et al. Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect. Immun. 74, 3554–3564 (2006).
Skare, J. T., Shaw, D. K., Trzeciakowski, J. P. & Hyde, J. A. In vivo imaging demonstrates that Borrelia burgdorferi ospC is uniquely expressed temporally and spatially throughout experimental infection. PLoS ONE 11, e0162501 (2016).
Hyde, J. A. et al. Bioluminescent imaging of Borrelia burgdorferi in vivo demonstrates that the fibronectin-binding protein BBK32 is required for optimal infectivity. Mol. Microbiol. 82, 99–113 (2011).
Weening, E. H. et al. BORRELIA BURGDORFERI lacking DbpBA exhibits an early survival defect during experimental infection. Infect. Immun. 76, 5694–5705 (2008).
Lin, Y.-P. et al. Strain-specific variation of the decorin-binding adhesin DbpA influences the tissue tropism of the Lyme disease spirochete. PLoS Pathog. 10, e1004238 (2014).
Shi, Y., Xu, Q., Seemanapalli, S. V., McShan, K. & Liang, F. T. The dbpBA locus of Borrelia burgdorferi is not essential for infection of mice. Infect. Immun. 74, 6509–6512 (2006).
Blevins, J. S., Revel, A. T., Smith, A. H., Bachlani, G. N. & Norgard, M. V. Adaptation of a luciferase gene reporter and lac expression system to Borrelia burgdorferi. Appl. Environ. Microbiol. 73, 1501–1513 (2007).
Purser, J. E. et al. A plasmid-encoded nicotinamidase (PncA) is essential for infectivity of Borrelia burgdorferi in a mammalian host. Mol. Microbiol. 48, 753–764 (2003).
Labandeira-Rey, M. & Skare, J. T. Decreased infectivity in Borrelia burgdorferi strain B31 is associated with loss of linear plasmid 25 or 28-1. Infect. Immun. 69, 446–455 (2001).
Maruskova, M., Esteve-Gassent, M. D., Sexton, V. L. & Seshu, J. Role of the BBA64 locus of Borrelia burgdorferi in early stages of infectivity in a murine model of Lyme disease. Infect. Immun. 76, 391–402 (2008).
Liveris, D. et al. Quantitative detection of Borrelia burgdorferi in 2-millimeter skin samples of erythema migrans lesions: correlation of results with clinical and laboratory findings. J. Clin. Microbiol. 40, 1249–1253 (2002).
Yang, X. et al. Interdependence of environmental factors influencing reciprocal patterns of gene expression in virulent Borrelia burgdorferi. Mol. Microbiol. 37, 1470–1479 (2000).
Pal, U. et al. Attachment of Borrelia burgdorferi within Ixodes scapularis mediated by outer surface protein A. J. Clin. Investig. 106, 561–569 (2000).
Pal, U. et al. TROSPA, an Ixodes scapularis receptor for Borrelia burgdorferi. Cell 119, 457–468 (2004).
Schwan, T. G. & Piesman, J. Temporal changes in outer surface proteins A and C of the lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J. Clin. Microbiol. 38, 382–388 (2000).
Hodzic, E., Feng, S., Freet, K. J. & Barthold, S. W. Borrelia burgdorferi population dynamics and prototype gene expression during infection of immunocompetent and immunodeficient mice. Infect. Immun. 71, 5042–5055 (2003).
Ouyang, Z. et al. Activation of the RpoN-RpoS regulatory pathway during the enzootic life cycle of Borrelia burgdorferi. BMC Microbiol. 12, 44 (2012).
Imai, D. M. et al. The early dissemination defect attributed to disruption of decorin-binding proteins is abolished in chronic murine Lyme borreliosis. Infect. Immun. 81, 1663–1673 (2013).
Blevins, J. S., Hagman, K. E. & Norgard, M. V. Assessment of decorin-binding protein A to the infectivity of Borrelia burgdorferi in the murine models of needle and tick infection. BMC Microbiol. 8, 82 (2008).
Xu, Q., Seemanaplli, S. V., McShan, K. & Liang, F. T. Increasing the interaction of Borrelia burgdorferi with decorin significantly reduces the 50 percent infectious dose and severely impairs dissemination. Infect. Immun. 75, 4272–4281 (2007).
Hyde, J. A. Borrelia burgdorferi keeps moving and carries on: a review of borrelial dissemination and invasion. Front. Immunol. 8, 114 (2017).
Aguero-Rosenfeld, M. E., Nowakowski, J., McKenna, D. F., Carbonaro, C. A. & Wormser, G. P. Serodiagnosis in early Lyme disease. J. Clin. Microbiol. 31, 3090–3095 (1993).
Boylan, J. A., Posey, J. E. & Gherardini, F. C. Borrelia oxidative stress response regulator, BosR: a distinctive Zn-dependent transcriptional activator. Proc. Natl. Acad. Sci. USA 100, 11684–11689 (2003).
Seshu, J. et al. A conservative amino acid change alters the function of BosR, the redox regulator of Borrelia burgdorferi. Mol. Microbiol. 54, 1352–1363 (2004).
Hyde, J. A., Seshu, J. & Skare, J. T. Transcriptional profiling of Borrelia burgdorferi containing a unique bosR allele identifies a putative oxidative stress regulon. Microbiology 152, 2599–2609 (2006).
Katona, L. I., Tokarz, R., Kuhlow, C. J., Benach, J. & Benach, J. L. The fur homologue in Borrelia burgdorferi. J. Bacteriol. 186, 6443–6456 (2004).
Dulebohn, D. P., Richards, C. L., Su, H., Lawrence, K. A. & Gherardini, F. C. Weak organic acids decrease Borrelia burgdorferi cytoplasmic pH, eliciting an acid stress response and impacting RpoN- and RpoS-dependent gene expression. Front. Microbiol. 8, 1734 (2017).
Ouyang, Z. & Zhou, J. BadR (BB0693) controls growth phase-dependent induction of rpoS and bosR in Borrelia burgdorferi via recognizing TAAAATAT motifs. Mol. Microbiol. 98, 1147–1167 (2015).
Drecktrah, D. et al. The Borrelia burgdorferi RelA/SpoT homolog and stringent response regulate survival in the tick vector and global gene expression during starvation. PLOS Pathog. 11, e1005160 (2015).
Karna, S. L. R. et al. CsrA modulates levels of lipoproteins and key regulators of gene expression critical for pathogenic mechanisms of Borrelia burgdorferi. Infect. Immun. 79, 732–744 (2011).
Miller, C. L., Karna, S. L. R. & Seshu, J. Borrelia host adaptation Regulator (BadR) regulates rpoS to modulate host adaptation and virulence factors in Borrelia burgdorferi. Mol. Microbiol. 88, 105–124 (2013).
Zhou, B., Yang, Y., Chen, T., Lou, Y. & Yang, X. F. The oligopeptide ABC transporter OppA4 negatively regulates the virulence factor OspC production of the Lyme disease pathogen. Ticks Tick-Borne Dis. 9, 1343–1349 (2018).
Mason, C., Liu, X., Prabhudeva, S. & Ouyang, Z. The CXXC motifs are essential for the function of BosR in Borrelia burgdorferi. Front. Cell. Infect. Microbiol. 9, 109 (2019).
Katona, L. I. The Fur homologue BosR requires Arg39 to activate rpoS transcription in Borrelia burgdorferi and thereby direct spirochaete infection in mice. Microbiology 161, 2243–2255 (2015).
Lybecker, M. C. & Samuels, D. S. Temperature-induced regulation of RpoS by a small RNA in Borrelia burgdorferi. Mol. Microbiol. 64, 1075–1089 (2007).
Lybecker, M. C., Abel, C. A., Feig, A. L. & Samuels, D. S. Identification and function of the RNA chaperone Hfq in the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 78, 622–635 (2010).
Drecktrah, D., Hall, L. S., Rescheneder, P., Lybecker, M. & Samuels, D. S. The stringent response-regulated sRNA transcriptome of Borrelia burgdorferi. Front. Cell. Infect. Microbiol. 8, 231 (2018).
Brown, E. L. et al. Resistance to Lyme disease in decorin-deficient mice. J. Clin. Investig 107, 845–852 (2001).
Ojaimi, C. et al. Profiling of temperature-induced changes in Borrelia burgdorferi gene expression by using whole genome arrays. Infect. Immun. 71, 1689–1705 (2003).
Liang, F. T. et al. Borrelia burgdorferi changes its surface antigenic expression in response to host immune responses. Infect. Immun. 72, 5759–5767 (2004).
Barbour, A. G. Isolation and cultivation of Lyme disease spirochetes. Yale J. Biol. Med. 57, 521–525 (1984).
Hyde, J. A., Weening, E. H. & Skare, J. T. Genetic transformation of Borrelia burgdorferi. Curr. Protoc. Microbiol. 20, 12C.4.1–12C.4.17 (2011).
Acknowledgements
We are grateful to Jon T. Skare and Magnus Hook for the BosR and DbpA anti-serum, respectively, used in this study. We thank Michael Norgard and Jon Blevins for providing the Borrelia codon optimized luciferase gene. We are appreciative to Geoffery Kapler and Raquel Sitcheran for generously sharing the Perkin Elmer Spectrum IVIS and resource necessary to complete this study. We are thankful to Vanessa Ante and Lauren Farris for assistance with this manuscript. This work was supported by NIAID Grant R21-AI101740-01.
Author information
Authors and Affiliations
Contributions
E.P.S. contributed to data acquisition and interpretation. J.P.T. performed statistical analysis. J.A.H. generated experimental design, performed data acquisition and interpretation, and wrote main manuscript text. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The author(s) declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saputra, E.P., Trzeciakowski, J.P. & Hyde, J.A. Borrelia burgdorferi spatiotemporal regulation of transcriptional regulator bosR and decorin binding protein during murine infection. Sci Rep 10, 12534 (2020). https://doi.org/10.1038/s41598-020-69212-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-69212-7
- Springer Nature Limited