Abstract
Road runoff carries a mixture of contaminants that threatens the quality of natural water bodies and the health of aquatic organisms. The use of sedimentation ponds is a nature-based solution for the treatment of road runoff. This study assessed the concentration of polycyclic aromatic hydrocarbons (PAHs) and their alkylated homologues in sediment from seven highway sedimentation ponds and three natural urban ponds. In addition, the study explored the bioaccumulation of PAHs in dragonfly nymphs (Anisoptera). Finally, biota-sediment accumulation factors (BSAFs) were estimated. The results revealed a significant difference in the concentrations of 16 priority PAHs in sediment, with overall higher levels in sedimentation ponds (2,911 µg/kg on average) compared to natural urban ponds (606 µg/kg on average). PAH levels increased substantially once alkylated homologues were considered, with alkylated comprising between 42 and 87% of the total PAH in sediment samples. These results demonstrate the importance of alkylated forms in the environmental assessment of PAHs. The bioaccumulation assessment indicates that dragonfly nymphs bioaccumulate PAHs to a certain degree. It is not clear, however, whether they metabolize PAHs. BSAF results ranged from approx. 0.006 to 10 and indicate that BSAFs can be a powerful tool to determine the functionality of sedimentation ponds.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
During traffic-related activities, a complex mixture of inorganic and organic contaminants is released into the environment. Some of these contaminants remain in the air or settle on the ground, being eventually washed off by rain1,2. Road runoff and tunnel wash water, enriched with contaminants, can eventually reach natural water bodies, threatening the water quality, and the health of all organisms dependent directly or indirectly on these systems.
Among many traffic-related contaminants, polycyclic aromatic hydrocarbons (PAHs) are probably the most studied group of organic contaminants. Tire and asphalt wear, and incomplete combustion of fuel are the main sources of PAHs from traffic3. PAHs are found in complex mixtures, with those of pyrogenic origin (from incomplete combustion) being mainly parent PAHs (without alkyl groups, heteroatoms or hydroxides), whereas those of petrogenic origin (from petroleum derivate) are associated with high levels of alkylated forms4.
Several PAHs and their metabolites are known to be potentially carcinogenic and mutagenic5,6,7, and some exhibit photo-induced toxicity8,9. Consequently, several PAHs have been added to the list of substances of concern in environmental risk assessment and monitoring. A list of 16 parent PAHs issued by the U.S. Environmental Protection Agency (EPA) in the 1970s, and referred in this study as PAH-16, is often used as a standard set of PAHs for environmental analysis10. A more recent study has, however, recommended a list of 40 PAHs when considering toxicity in the environment, including several alkylated homologues11. Some alkylated PAHs are reported to be more toxic than their parental forms12, 13.
A variety of treatment solutions can be established to reduce the impacts of PAHs and other traffic related contaminants present in road runoff on water bodies. One of the most commonly used mitigation methods is the use of nature-based sedimentation ponds. These low-cost, sustainable urban drainage systems are built to retain particle-bound contaminants, improving the quality of the runoff before its discharge into natural water bodies14. Quite rapidly, these ponds are colonized by a wide range of organisms, leading to the formation of small complex ecosystems in rather urban zones15,16. These systems’ apparent ability to support and enhance biodiversity is often acknowledged as a positive ecosystem service by, e.g. road and urban planners. However, some have argued that biota inhabiting such nature-based sedimentation ponds can be harmed by the contaminants they are inevitably exposed to in these systems17,18. For example, our research group recently documented increased genotoxicity in nymphs of dragonflies (Odonata: Anisoptera, Aeshna sp.) living in sedimentation ponds compared to those living in natural ponds19. Whereas vertebrates metabolize PAHs20,21,22,23, metabolism efficiency varies among invertebrates24,25,26. Furthermore, PAHs may potentially be transported out of the ponds by organisms with both aquatic and terrestrial life stages. Dragonflies have an aquatic life stage that can last years, followed by a short, terrestrial, adult life stage. They are, therefore, potential vectors of bioaccumulative contaminants from the aquatic to terrestrial environments.
In the present study, we determined the concentration of PAHs and their alkylated homologues in sediment, and assessed bioaccumulation and biotransformation of PAHs in dragonfly nymphs from the genus Aeshna. Aeshna nymphs spend most of their time hidden between the aquatic vegetation where they prey on various invertebrates and small vertebrates such as tadpoles. Sediment and dragonfly nymphs from seven nature-based sedimentation ponds and three natural urban ponds were included in the study.
Results
Levels of PAHs and their estimated alkylated homologues in sediment
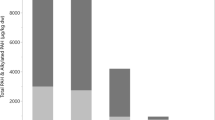
The levels of individual parental and estimated alkylated PAHs are shown in Table 1. Ponds were grouped as SED—n for sedimentation ponds and NAT—n for natural urban ponds. All sites are described in Table 2. Analysis of the concentrations of PAHs in sediment samples showed overall higher concentrations of PAH-16 in sedimentation compared to natural urban ponds (Fig. 1a—Welch’s Two Sample t-test, p = 0.025). The exceptions were the sedimentation ponds SED—5 and 7, in which PAH levels were lower than in some of the natural urban ponds. The highest levels of PAHs were detected in the samples from SED—1, 2, 4, and 6. Inclusion of alkylated PAHs led to a substantial increase in total PAH levels (Fig. 1b—Welch’s Two Sample t-test, p = 0.002). All sedimentation ponds had higher concentrations of total PAHs compared to natural urban ponds once alkylated forms were included.
PAH-16 levels in the sediments were classified according to the environmental quality standards (EQSs) set by the Norwegian Committee of Directorates for the Water Framework Directive27. The classification ranges from I to V, according to the PAH concentrations. Exposure to PAH concentrations classified as levels I or II are considered low, and no toxic effects are expected. Exposure to levels III and IV might cause chronic and acute effects in organisms, respectively 28. Many of the PAHs detected in our sediment samples were classified as III and IV (Fig. 2). Inclusion of alkylated results in the concentrations of phenanthrene, naphthalene, fluorene, and chrysene caused these PAHs to change their classification from class II to class IV in several cases (Fig. 3).
Concentrations of PAH-16 (a), and PAH-16 and alkylated PAHs (b) in sediment samples from sedimentation and natural urban ponds. Concentration in µg/g (dry weight). Black horizontal line represents the median. Asterisk represents the mean. Each data point represents a pond. Natural, n = 3. Sedimentation, n = 7. PAHs below the detection limit were set to ½ the detection limit value.
Bar plot of the classification of PAH-16 per sediment sample (n-16) according to the EQS set by the Norwegian Committee of Directorates for the Water Framework Directive27.
Bar plot of the classification of PAHs phenanthrene, naphthalene, fluorene, chrysene, and their alkylated PAHs per pond, excluding NAT-3 (n = 9). EQS set by the Norwegian Committee of Directorates for the Water Framework Directive27.
PAHs in dragonfly nymphs
Concentrations
Concentrations of parent PAHs in tissues, exuvia, and whole dragonfly nymphs were determined in order to investigate bioaccumulation (Supplementary table S1). PAHs acenaphthene, acenaphthylene, phenanthrene, fluoranthene, and pyrene were detected in at least 80% of all samples, and for that reason they were the only ones used in the following statistical analyses. Early instars were not found in NAT—2, and thus not included.
A comparison between the concentrations of PAHs in nymphs from sedimentation and natural urban ponds did not reveal a significant difference between groups (Fig. 4a—Welch’s Two Sample t-test, p-value = 0.2). The result was caused by particularly high concentrations of phenanthrene, fluoranthene, and pyrene in some of the Late instars from NAT—1.
Boxplots of the sum of the concentration of PAHs in nymphs from sedimentation and natural urban ponds (a), early and late instars (b), and exuvia and tissues of late instars (c). Concentration in ng/g (dry weight). Black horizontal line represents the median. Asterisk represents the mean. Late, n = 10, Early, n = 9. Exuvia: n = 10, Tissue; n = 1.
A comparison between PAH concentrations in early instars and late instars nymphs, independent of location, showed significantly higher levels in late instar nymphs in comparison to early instars (Fig. 4b—Welch’s Two Sample t-test, p = 0.04). There was also a significant difference between exuvia and tissues sampled from the same nymphs, with higher levels of PAHs in the tissues (Fig. 4c—Welch’s Two Sample t-test, p = 0.007).
PAH metabolites
Levels of 1-OH-phenanthrene, 1-OH-pyrene and 3-OH-benzo[a]pyrene were analysed in nymph haemolymph. Only 1-OH-pyrene was detected, at very low levels, and only in samples from some of the ponds (Fig. 5, Supplementary table S2).
A Pearson correlation analysis revealed no statistically significant relationship between 1-OH-pyrene concentrations and pyrene concentrations (calculated using the sum of concentration in tissue and exuvia) in late instar nymphs (r = − 0.21, p = 0.55, n = 10).
Biota-sediment bioaccumulation factors (BSAF)
BSAF results revealed ratios ranging from approx. 0.006 to 10 (Fig. 6), with a significant difference between PAHs (Kruskal–Wallis, p < 0.001). The highest BSAF values were of acenaphthene and acenaphthylene, followed by phenanthrene. The lowest BSAFs were of pyrene and fluoranthene. Most BSAFs from sedimentation ponds were below 1, whereas in natural urban ponds most BSAFs were above 1. Linear regression analysis showed a strong negative relationship between the mean of BSAFs and Kow (R2 = 0.91, p = 0.007; Fig. 7).
Discussion
Levels of PAHs and their alkylated homologues in sediment
The results showed a significant difference in concentrations of PAH-16 in sediment, with higher levels being detected in the sedimentation ponds. Consequently, the biota living in these artificial ponds are exposed to higher levels of PAHs than biota living in the ponds that are not directly affected by highway runoff. Previous studies have shown that traffic-related contaminants can lead to negative effects in aquatic organisms inhabiting sedimentation ponds. Examples are fish3,36, insects53, and amphibians37. Therefore, sedimentation ponds may represent a low-quality habitat compared to natural urban ponds. Regression analysis suggested a relationship between the concentrations of PAHs in sedimentation ponds and annual average daily traffic (AADT); R2 = 0.61, p = 0.03. The results, however, were highly influenced by SED-7.
Samples from SED—5 and 7 contained the lowest concentrations amongst all sedimentation ponds. PAH-16 concentrations in sediment from SED—7 were exceptionally low. A possible reason for such low concentrations might be that these ponds are not functioning properly, and hence, they may either not be receiving the runoff or not retaining contaminants as predicted. Further examination of these ponds should be considered to investigate their functionality.
In this study we took a step further from the standard analysis of PAH-16 only. The addition of alkylated PAHs to the total sum of PAHs led to a significant increase in measured PAH levels in virtually all sediment samples. The use of a limited list of PAHs as a proxy reduces costs and analytical complexity, which can result in better comparability between analyses performed worldwide11. Several studies indicated, however, the effects of alkylated PAHs in aquatic organisms, such as growth inhibition, malformation, reduction of survival rates38, and behavioral disruption39. A toxicity test performed by Turcotte et al. 13 revealed that alkylated forms of phenanthrene were more toxic than its parental form, and that toxicity increased with increase of alkyl substituents. PAHs of petrogenic origin often contain high levels of alkylated PAHs in relation to their parental forms. Therefore, analysis of only parent forms might potentially ignore much higher concentrations of the total PAHs in many situations, distorting the true environmental status, and the potential threat from these contaminants. Based on our results, analysis of a more extensive list of PAHs in environmental samples, as suggested by Andersson & Achten11, showed to be useful for road runoff contaminated sites.
PAHs in dragonfly nymphs
Only parent PAHs and metabolites were analysed in the nymph samples as the separation of exuvia and tissue in late nymphs limited the amount of material available. PAHs were detected in the exuvia, indicating that dragonfly nymphs are able to eliminate PAHs through molting. The results showed, however, a significant difference in the concentration of PAHs in the tissue and exuvia, with over 65% of total PAHs being detected in tissue. Concentrations of PAHs in Early and Late instars were also significantly different, and higher concentrations were detected in the latter group. Hence, despite the nymph’s capacity to eliminate PAHs through molting, the level of elimination is likely not enough to avoid bioaccumulation.
To the best of our knowledge, this is the first study investigating the biotransformation of PAHs in dragonflies. 1-OH-pyrene was the only metabolite detected, at very low levels, and only in some samples. Since 1-OH-pyrene was the most abundant PAH metabolite found in previous analyses conducted by our research group, it was not a surprise that no other metabolite was detected in the present study. Parent phenanthrene was detected at higher levels in most tissue samples compared the other PAHs found, whilst no metabolites were detected. These results suggest that phenanthrene was not metabolized by the individuals used in this study. It is therefore not clear whether 1-OH-pyrene found in our samples came from external sources or if metabolism of PAHs in dragonfly nymphs is too inefficient to avoid a certain level of bioaccumulation.
The low levels of 1-OH-pyrene detected could also be the result of trophic transfer. Previous studies observed uptake of PAH metabolites through diet40,41,42,43. Due to invertebrates’ ineffective metabolic processes, metabolites may take longer to be biotransformed from phase I to phase II, thus remaining longer in the prey’s body. In addition, invertebrates excrete compounds slower than vertebrates due to the lack of kidneys44. Thus, metabolic PAHs may remain in invertebrate prey long enough to be transferred to predators. Nevertheless, dragonfly nymphs are efficient predators, and vertebrates such as tadpoles are also included in their diet45,46. Tadpoles were spotted in almost all ponds included in this study. Macroinvertebrates biotransform PAHs at different efficiency levels24,25,26,47. A better understanding of the diet of the nymphs in these ponds could perhaps explain why 1-OH-pyrene were detected in some samples, but not in others. The potential trophic transfer is of concern due to the toxic nature of PAH metabolites.
Despite our results suggesting bioaccumulation of PAHs in dragonfly nymphs, it is possible that these contaminants are not transferred from the nymph/aquatic to the adult/terrestrial stage. Wayland et al. 48 observed similar levels of PAHs in damselfly adults and nymphs (Odonata: Zygoptera). Lower levels would be expected in the nymphs if bioaccumulation was a factor. The study suggests that bioaccumulation of PAHs in adult damselflies’ might be mostly due to the insect’s feeding habits, and less from possible bioaccumulation during their aquatic life stage. In addition, a study from Kraus et al. 49 on biomagnification of contaminants in insects demonstrated a great loss of PAHs during metamorphosis, with nymph concentrations being up to 125-fold higher than in adults. As hemimetabolous species, dragonflies go through a major transition during metamorphosis. Their aquatic and terrestrial life stages are associated with completely different lifestyle and appearance. While their nymph stage specializes in growth, their adult life is specialized for dispersal and reproduction50. It is, therefore, reasonable to suggest that bioaccumulation might occur only during the dragonfly’s nymph stage.
Biota-sediment bioaccumulation factors (BSAF)
This study observed BSAF values as low as 0.006 and as high as 10. Our results may, however, be subject to uncertainty since we were unable to determine lipid content for nymphs from individual sites due to limited material available. Nevertheless, as our samples are most likely of the same species, and we grouped the individuals according to similar size and weight, we do not expect much variation in lipid content based on site location. In addition, previous analysis performed by our team in nymphs from individual sites (including some sites used in this study) showed a lipid variation of only 1.6% (unpublished results).
Our results showed that BSAFs decreased with increase of PAH’s Kow, in line with previous studies51,52, and the correlation between BSAF and partition coefficient has been suggested as a BSAF predictor for bottom dwellers53. Virtually all 5- and 6-ring PAHs in dragonfly nymphs were below quantification levels. This was not a surprise, as heavier PAHs tend to strongly adsorb to sediment54,55. The only 4-ring PAHs quantified were pyrene and fluoranthene, which have Kow values below 5.5. Thus, our results suggest that less hydrophobic PAHs (i.e. those with relatively low Kow) might not adsorb strongly enough to sediment at certain conditions, being as a result more bioavailable. Low molecular weight PAHs might be dissolved in water or adhered to suspended particles (< 62 µm). Since surface area is inversely proportional to particle size, smaller particles have a higher contaminant-carrying capacity than larger particles56,57. In an experiment in which the distribution of PAHs from road runoff was quantified in particulate fractions, Nielsen et al. 58 detected a significant amount of low- and middle molecular weight PAHs in the colloidal and dissolved fractions in traffic runoff.
BSAF for PAHs are often reported in bottom-dwelling filter feeders. Nonetheless, Aeshna species might be highly exposed to sediment as they live very close to the bottom, and most likely include bottom-dwelling organisms in their diet. In addition, their gills are placed inside their rectal chamber; thus water, and unavoidably sediment particles, are pumped inside their bodies during respiration59.
Pyrene, fluoranthene, and phenanthrene were accounted for the most part of the observed toxicity in freshwater amphipods exposed to highway runoff in tests that also included anthracene, chrysene, and benzo[a]anthracene60. Carls et al. 61 demonstrated the toxic effects of dissolved petrogenic PAHs by exposing fish embryos indirectly to oil droplets that were kept isolated by using an agarose barrier. Thus, analysis of PAHs in water and suspended particles should be considered in future studies to investigate the bioavailability of these organic compounds.
The BSAF/Kow relationship might, however, be due to other factors other than the adsorption capacity of PAHs. For instance, Thomann and Komlos52 observed that low BSAFs for PAHs with Kow > 5 in sunfish (also a predator) were primarily due to low gut assimilation capacity and high metabolism efficiency.
BSAF values were generally higher in natural urban ponds compared to sedimentation ponds. This could be the result of the selected PAHs being more bioavailable in natural urban ponds. Previous studies have shown that BSAFs may be influenced by physical–chemical characteristics of the sediment62, ecological characteristics63, and the contaminant’s Kow 64. BSAFs might therefore be a useful tool to indicate the contaminant-retaining functionality of the sedimentation ponds. To support this contention, the sedimentation ponds with the highest overall BSAF ratios were the same as those with particularly low PAH concentration detected in sediments (SED—5 and 7). Nevertheless, Meland et al. 65 observed higher levels of DNA damage in dragonfly nymphs (Aeshna sp.) living in sedimentation ponds compared to natural urban ponds. In addition, a strong correlation between levels of DNA damage and PAH levels in sediment was observed in the same study.
PAHs were detected in significant higher levels in dragonfly nymph tissues than in exuvia, indicating some level of bioaccumulation of PAHs in dragonfly nymphs. In addition, it is not clear whether dragonfly nymphs biotransform PAHs inefficiently, or if the 1-OH-pyrene detected was a product of trophic transfer. Further studies would be needed to answer this question.
Our results showed that the inclusion of alkylated PAHs drastically increased the overall measured PAH levels in sediments in all ponds, and thus gave a more realistic picture of the status of the ponds studied. Previous studies have observed that BSAF for alkylated PAHs are often > 166, and in some cases they were greater than for parent PAHs with similar Kow 52, including for dragonfly nymphs48. In this study we determined BSAF for parent PAHs only. As our results suggest, alkylated PAHs comprise a large percentage of the overall PAH in sediments contaminated by road runoff. It is therefore important to include alkylated homologues when determining BSAF from species exposed to such sediments.
Sedimentation ponds are oases for research on the effects and fate of contaminants in the environment. It is crucial, however, that their ecological role is considered during the design and construction process to enhance their capacity as suitable habitats, and ensure that they remain an environmental solution instead of an environmental burden.
Materials and methods
Study sites
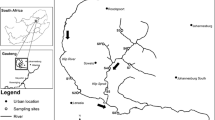
Nymphs and sediment samples were collected from seven sedimentation ponds and three natural urban ponds situated in the counties of Oslo, Akershus, and Østfold, in Norway (Fig. 8). Sedimentation ponds were labelled as SED—1 to 7, and natural ponds as NAT -1 to 3 (Table 2).
Map showing the location of the ponds. Sedimentation ponds are marked with circles and natural urban ponds are marked with triangles. Map was obtained from the Norwegian Mapping Authority’s online map service (www.norgeskart.no).
Sampling
Samples were collected in June 2018, except for the early instars and sediment from NAT—3, which were collected in June 2017. All samples were transported to the laboratory in glass jars sterilized at 550 °C for 30 min. Sediment samples were collected from approximately the top 5 cm of each location with a van Veen grab. Each sediment sample consisted of material collected from five different spots which were combined and mixed. Nymphs were transported in polystyrene boxes with ice. Once at the laboratory, nymphs were rinsed with distilled water, pat dried, and killed by introducing a scalpel to the head. They were then weighed and measured (Table 3). Nymphs were identified to be from the Genus Aeshna, as described by Brooks & Cham67. Individuals were divided into groups Early instars and Late instars (supplementary figure S1). Late instars were inferred to be individuals in instars F1–F0 (penultimate and final instars, respectively), and Early instars were those in earlier developmental stages. Stages were determined by using the minimum and maximum size values for body and wing pad length for Aeshna cyanea as determined by Goretti et al. 68. Samples were stored in sterilized jars (550 °C for 30 min), except for haemolymph, which was kept in glass capillaries. Sampled materials were pooled in order to obtain enough material to detect contaminants (Table 3), and kept at − 20 °C until further analysis.
PAH determination
Extraction and clean-up
PAHs were analysed in all samples. Due to limited biota material, alkylated homologues were analysed only in sediment. Nymphs in the Late instars group had exuvia and tissue analysed separately, and haemolymph extracted for determination of PAH metabolites. Early instars were analysed as a whole. Exuvia and the internal tissues were separated in frozen individuals. Internal tissues were scraped out with a metal spoon. Wing pads were pulled off to get access to the wings, which were already developed in some individuals. Tissue and exuvia were transferred to separately marked extraction glasses for further analysis.
Approximately 5 g of sediment and 2–8 g of nymphs (split between tissues and exuvia if late instars) were freeze-dried for 48 h. Dried contents were homogenized with a glass stirring rod and mixed with approximately 15 mL of cyclohexane: dichloromethane (90:10). 50 µL of PAH internal standard (2 µg/mL, dissolved in toluene) were added. The internal standards were naphthalene-d8, biphenyl-d10, acenaphthylene-d8, dibenzothiophene-d10, pyrene-d10, benzo(a)anthracene-d12, and perylene-d12. The following procedure was done twice, and extracts combined: The extracts were placed in an ultrasonic bath for 1 h and centrifuged for 5 min at 3,000 RPM. Extracts were concentrated to 1 mL in an automated solvent evaporation system (TurboVap LV) at 37 °C, transferred to 2 mL vials, and further concentrated to approx. 100 µL. Next, 400 µL of ethyl acetate (LS-MS graded) was added. Extracts were transferred to polypropylene microcentrifuge tubes with centrifuge tubes filters (0.2 µM nylon filters), centrifuged for 1 min at 13,000 RPM, and transferred to vials. A small amount of cyclohexane was added, and the extracts were further concentrated by a gentle flow of nitrogen to approximately 100 µL before being transferred to 0.9 mL vials for analysis.
Biota extract was cleaned by Gel Permeation Chromatography (Agilent Technologies, Wilmington, DE, USA), with a 300 × 7.5 mm column of the type PLgel 10 um, 100 Å (pore size). Fraction collected was between 4.8–11.3 µL/min at 50 °C. Mobile phase consisting of ethyl acetate: cyclohexane 80:20, and flow of 2 mL/min was used. Sediment and biota extracts were analysed by gas chromatography/mass spectrometry operated in single ion monitoring mode (SIM—Agilent GC 6,890/MSD 5,973; Agilent Technologies, Wilmington, DE, USA). The internal standard method was used for quantification of individual components. LODs were determined by the “signal to noise” method as described by Shrivastava and Gupta69.
GC/MS analysis
The analyses were performed as described in Meland et al. 65. In brief, we used gas chromatography/mass spectrometry (Agilent GC 6890/MSD 5973; Agilent Technologies, Wilmington, DE, USA) operated in single ion monitoring mode (SIM). The ionization was electron impact (70 eV). PAHs were individually separated on a DB5 column (30 m, 0.25 mm inner diameter, and 0.25 µm film thickness: Agilent JW Scientific). The injection was pulsed splitless injection (2 µL injection, pulse pressure 20 psi for 1.2 min, injection temperature 300 °C), and the carrier gas was He (1.2 mL/min). The temperature of the GC oven started at 60 °C for the first 2 min, then further raised to 250 °C (7 °C/min), and was finally raised to 310 °C (15 °C/min), being kept at that temperature for 6 min. Temperatures for the quadrupole, ion source, and transfer line were 150 °C, 230 °C, and 300 °C respectively. Concentrations of alkylated PAHs were estimated using the response factors of the corresponding parent PAH. Limit of detection (LOD) ranged from 2 to 1,100 µg/kg for sediment, and 1 to 30 ng/g for biota.
PAH metabolites
Extraction and clean-up
Haemolymph was extracted by removing the middle leg of the nymphs and applying gentle pressure for its release into a glass capillary (Hilgenberg, 80 mm length, 0.4 mm outer circumference, 0.04 mm wall thickness). The volume of haemolymph extracted was measured by its weight. Approximately 10 µL of haemolymph were transferred to polypropylene microcentrifuge tubes, followed by 10 µL (1,000 µg/mL) of internal standard; triphenylamine. During the procedure, the samples were kept on ice whenever possible, whilst kept away from direct light. 50 µL of Milli-Q water (18.2 MΩ·cm resistivity with Millipak membrane filter 0.22 µm; Merck KGaA, Germany) was added to the samples, followed by 20 µL of β-glucuronidase (200 U/µL haemolymph) with aryl sulfatase activity (10 U/µL haemolymph). Samples were set on a heating block at 37 °C for 1 h, and then 200 µL of methanol was added and mixed well. Samples were centrifuged for 10 min at 13,000 RPM, and supernatants were transferred to 300 µL vials and kept in a freezer at − 20 °C until analysis.
HPLC analysis
Metabolite analysis was performed as described in Grung et al. 3,36. Samples containing 25 µL of haemolymph extract were analysed by high performance liquid chromatography (HPLC) using Waters 2,695 Separations Module with a Waters PAH C18 (4.6 × 250 mm, 5 µm) column and 2,475 fluorescence detector. Calibration standards from Chiron AS, Trondheim, Norway (0.2–200 ng/g. range), and internal standard method were used for quantification of individual components. Mobile phase was comprised of a gradient starting from 40:60 acetonitrile : ammonium acetate aqueous solution (0.05 M, pH 4.1) to 100% acetonitrile gradient at a flow of 1 mL/min (total time of 60 min), and column temperature of 35 °C. Fluorescence excitation/emissions: 1-OH-phenanthrene 256/380; 1-OH-pyrene 346/384; triphenylamine 300/360; 3-OH-benzo[a]pyrene 380/430). LOD ranged between 0.1 and 3 ng/g (0.18 ng/g bile for 1-OH-pyrene, which is the PAH metabolite most often detected). This is quite low in comparison with many analyses of PAH-metabolites performed by us in bile from fish, where the LOD were up to 5 times this level36. Fish bile, however, generally contains a significant number of compounds that might cause chromatographic noise. There was less background noise in the haemolymph chromatograms compared to bile, thereby facilitating a lower LOD than usual in our lab. Since PAHs are detected in the nymphs, we were expecting low or non-detectable levels of PAH-metabolites. We have also previously analysed blue mussels (Mytilus edulis; unpublished results), and, as in the case of dragonfly nymphs used in this study, PAH-metabolites were detected at very low levels. Blue mussels are species known for their accumulation of PAHs and are used as sentinel organisms in monitoring of oil pollution.
Biota-sediment accumulation factor (BSAF)
BSAF is the ratio of the concentration of a contaminant in the organism and sediment. BSAF for each PAH was measured in early and late instar samples using the following equation:
where Cbiota is the concentration of PAH in the biota (early and late instars, mg/kg, dry weight), flip is the fraction of lipid content in the biota (mg/kg, dry weight), Csed is the concentration of PAH in the sediment (mg/kg, dry weight), and foc is the fraction of organic content in the sediment (mg/kg, dry weight). flip and foc were analysed by the Department of Organic Chemistry at NIVA. flip was determined by pooling same-size nymphs from all ponds (one fraction for early and one for late instars). BSAF for NAT2 was not calculated because we did not have foc for this sediment sample.
For benthic organisms, partition equilibrium of a chemical between sediment and organism is expected at BSAF values between 1 and 270.
Quality assurance
Quality assurance was performed as described by Meland et al. 65. In short, we analysed two samples of Standard Reference Material (SRM) and three blank samples (containing internal standards) with every batch in order to trace potential sample contamination or loss of contaminants. For sediment analysis we used NIST SRM 1944 with an average deviation of − 11% (min. − 32%, max. 9%). For PAH analysis in nymphs NIST SRM 2974a was used, with average deviation of − 12.7% (min. − 2.8%, max. − 20.3%). For metabolite analysis we used reference material prepared at NIVA, and the average deviation was of − 4.5% (min. − 62%, max. 25%).
Statistical analysis and data handling
Statistical analyses were performed using RStudio (version 1.1.456-2009-2018) and JMP software (SAS Institute, version 14.0.0-2018).
For dragonfly data, only PAHs which had at least 80% of the samples quantified were used. Observations reported as “less than” ( <) were substituted with half of its value. For sediment and haemolymph data, different substitution methods (as described in Wood, Beresford & Copplestone71) were tested, with no significant difference in the overall results. Consequently, [( <)/2] was also applied for variables containing less than 80% of observations detected, when appropriate.
Significance level was set to α = 0.05 for all statistical tests. Shapiro–Wilk tests for normal distribution were performed. Data were log-transformed when normality assumptions were not met. Welch's t-test was performed whenever the normality assumption was met. The Spearman's rank-order was used for correlation analysis.
Data availability
Datasets are available from the authors upon request.
References
Ngabe, B., Bidleman, T. F. & Scott, G. I. Polycyclic aromatic hydrocarbons in storm runoff from urban and coastal South Carolina. Sci. Total Environ. 255(1), 1–9 (2000).
Mangani, G. et al. Evaluation of the pollutant content in road runoff first flush waters. Water Air Soil Pollut. 160(1), 213–228 (2005).
Meland, S., Salbu, B. & Rosseland, B. O. Ecotoxicological impact of highway runoff using brown trout (Salmo trutta) as an indicator model. J. Environ. Monit. 12(3), 654–664 (2010).
Wang, Z. et al. A case study: distinguishing pyrogenic hydrocarbons from petrogenic hydrocarbons. Int. Oil Spill Conf. Proc. 2008(1), 311–320 (2008).
Aas, E. et al. PAH metabolites in bile, cytochrome P4501A and DNA adducts as environmental risk parameters for chronic oil exposure: a laboratory experiment with atlantic cod. Aquat. Toxicol. 51(2), 241–258 (2000).
Shaw, G. R. & Connell, D. W. DNA adducts as a biomarker of polycyclic aromatic hydrocarbon exposure in aquatic organisms: relationship to carcinogenicity. Biomarkers 6(1), 64–71 (2001).
Penning, T. M. Dihydrodiol dehydrogenase and its role in polycyclic aromatic hydrocarbon metabolism. Chem. Biol. Interact. 89(1), 1–34 (1993).
Newsted, J. L. & Giesy, J. P. Predictive models for photoinduced acute toxicity of polycyclic aromatic hydrocarbons to Daphnia magna, Strauss (Cladocera, Crustacea). Environ. Toxicol. Chem. 6(6), 445–461 (1987).
Bowling, J. W. et al. Acute mortality of anthracene-contaminated fish exposed to sunlight. Aquat. Toxicol. 3(1), 79–90 (1983).
Keith, L. H. The source of U.S. EPA’s sixteen PAH priority pollutants. Polycycl. Aromat. Compd. 35(2–4), 147–160 (2015).
Andersson, J. T. & Achten, C. Time to say goodbye to the 16 EPA PAHs? Toward an up-to-date use of PACs for environmental purposes. Polycycl. Aromat. Compd. 35(2–4), 330–354 (2015).
Marvanova, S. et al. Toxic effects of methylated benz[a]anthracenes in liver cells. Chem. Res. Toxicol. 21(2), 503–512 (2008).
Turcotte, D. et al. Measuring the toxicity of alkyl-phenanthrenes to early life stages of medaka (Oryzias latipes) using partition-controlled delivery. Environ. Toxicol. Chem. 30(2), 487–495 (2011).
Meland, S. Management of Contaminated Runoff Water: Current Practice and Future Research Needs. CEDR report (Conference of European Directors of Roads (CEDR). Brussels, 2016, p. 84).
Le Viol, I. et al. The contribution of motorway stormwater retention ponds to the biodiversity of aquatic macroinvertebrates. Biol. Cons. 142(12), 3163–3171 (2009).
Sun, Z. et al. Impact of environmental factors on aquatic biodiversity in roadside stormwater ponds. Sci. Rep. 9(1), 5994 (2019).
Snodgrass, J. et al. Microcosm investigations of stormwater pond sediment toxicity to embryonic and larval amphibians: variation in sensitivity among species. Environ. Pollut. (Barking, Essex: 1987) 154, 291–297 (2008).
Bishop, C. A. et al. Contamination and wildlife communities in stormwater detention ponds in Guelph and the greater Toronto area, Ontario, 1997 and 1998 Part II—contamination and biological effects of contamination. Water Qual. Res. J. 35(3), 437–474 (2000).
Meland, S. et al. Road related pollutants induced DNA damage in dragonfly nymphs (Odonata, Anisoptera) living in highway sedimentation ponds. Sci. Rep. 9(1), 16002 (2019).
Giessing, A. M. B., Mayer, L. M. & Forbes, T. L. Synchronous fluorescence spectrometry of 1-hydroxypyrene: a rapid screening method for identification of PAH exposure in tissue from marine polychaetes. Mar. Environ. Res. 56(5), 599–615 (2003).
Grung, M. et al. Polycyclic aromatic hydrocarbon (PAH) metabolites in atlantic cod exposed via water or diet to a synthetic produced water. J. Toxicol. Environ. Health Part A 72(3–4), 254–265 (2009).
Sundt, R. C. et al. Biomarker responses in atlantic cod (Gadus morhua) exposed to produced water from a north sea oil field: laboratory and field assessments. Mar. Pollut. Bull. 64(1), 144–152 (2011).
Whyte, J. J. et al. Ethoxyresorufin-O-deethylase (EROD) activity in fish as a biomarker of chemical exposure. Crit. Rev. Toxicol. 30(4), 347–570 (2000).
Van Brummelen, T. C. et al. Polycyclic aromatic hydrocarbons in earthworms and isopods from contaminated forest soils. Chemosphere 32(2), 315–341 (1996).
Stroomberg, G. J. et al. PAH biotransformation in terrestrial invertebrates—a new phase II metabolite in Isopods and springtails. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 138(2), 129–137 (2004).
Rust, A. J. et al. Relationship between metabolism and bioaccumulation of Benzo[α]pyrene in benthic invertebrates. Environ. Toxicol. Chem. 23(11), 2587–2593 (2004).
Committee of Directorates for the Water Framework Directive, Veileder 2:2018 Klassifisering av Miljøtilstand i Vann - Økologisk og Kjemisk Klassifiseringssystem for Kystvann, Grunnvann, Innsjøer og Elver (In Norwegian) (eds Vannforskriften D.f.G.a.), (Norway, 2018).
Norwegian Environmental Agency, Quality Standards for Water, Sediment and Biota (In Norwegian) 24 (Norway 2016).
Stogiannidis, E. and R. Laane, Source Characterization of Polycyclic Aromatic Hydrocarbons by Using Their Molecular Indices: An Overview of Possibilities, in Reviews of Environmental Contamination and Toxicology (edsWhitacre, D.M.) 49–133 (Springer International Publishing, Cham, 2015).
Lerda, D., Polycyclic Aromatic Hydrocarbons (PAHs) Factsheet (eds Measurements, J.R.C.I.f.R.M.a.) 34 (European Union, Belgium, 2011).
NCBI, N.C.f.B.I., PubChem Compound Database. National Center for Biotechnology Information.
National Center for Biotechnology Information. Tert-butyl Methyl Ether. PubChem Compound Database; CID=15413 [cited 2018 Jan. 20]; Available from: https://pubchem.ncbi.nlm.nih.gov/compound/15413.
Stout, S. A., Uhler, A. D. & Emsbo-Mattingly, S. D. Comparative evaluation of background anthropogenic hydrocarbons in surficial sediments from Nine Urban waterways. Environ. Sci. Technol. 38(11), 2987–2994 (2004).
Agency for Toxic Substances and Disease Registry, Toxicological Profile for Polycyclic Aromatic Hydrocarbons (PAHs). 1995, U.S. Department of Health and Human Services, Public Health Service.: Atlanta, GA.
European Commission. Priority Substances and Certain Other Pollutants according to Annex II of Directive 2008/105/EC. 2008 08.06.2016 [cited 05.08.2018; Available from: https://ec.europa.eu/environment/water/water-framework/priority_substances.htm.
Grung, M. et al. PAH related effects on fish in sedimentation ponds for road runoff and potential transfer of PAHs from sediment to biota. Sci. Total Environ. 566–567, 1309–1317 (2016).
Brand, A. B. et al. Lethal and sublethal effects of embryonic and larval exposure of hyla versicolor to stormwater pond sediments. Arch. Environ. Contam. Toxicol. 58(2), 325–331 (2010).
Vignet, C. et al. Chronic dietary exposure to pyrolytic and petrogenic mixtures of PAHs causes physiological disruption in Zebrafish—Part I: survival and growth. Environ. Sci. Pollut. Res. 21(24), 13804–13817 (2014).
Vignet, C. et al. Chronic dietary exposure to pyrolytic and petrogenic Mixtures of PAHs causes physiological disruption in Zebrafish—part II: behavior. Environ. Sci. Pollut. Res. 21(24), 13818–13832 (2014).
Carrasco Navarro, V. et al. Trophic transfer of pyrene metabolites between aquatic invertebrates. Environ. Pollut. 173, 61–67 (2012).
Beach, D. G. & Hellou, J. Bioaccumulation and biotransformation of 1-hydroxypyrene by the marine Whelk Neptunea lyrata. Int. J. Environ. Anal. Chem. 91(13), 1227–1243 (2011).
McElroy, A. E. et al. Relative bioavailability and DNA adduct formation of Benzo[a]pyrene and metabolites in the diet of the winter flounder. Comp. Biochem. Physiol. C 100(1–2), 29–32 (1991).
Carrasco Navarro, V. et al. Trophic transfer of pyrene metabolites and nonextractable fraction from Oligochaete (Lumbriculus variegatus) to Juvenile Brown Trout (Salmo trutta). Chemosphere 88(1), 55–61 (2012).
Lyytikäinen, M. et al. Bioaccumulation and biotransformation of polycyclic aromatic hydrocarbons during sediment tests with Oligochaetes (Lumbriculus variegatus). Environ. Toxicol. Chem. 26(12), 2660–2666 (2007).
McCollum, S. A. & Leimberger, J. D. Predator-induced morphological changes in an amphibian: predation by dragonflies affects tadpole shape and color. Oecologia 109(4), 615–621 (1997).
Laurila, A., Kujasalo, J. & Ranta, E. Different antipredator behaviour in two anuran tadpoles: effects of predator diet. Behav. Ecol. Sociobiol. 40(5), 329–336 (1997).
Carrasco Navarro, V. PAHs: Comparative Biotransformation and Trophic Transfer of Their Metabolites in the Aquatic Environment : Fate of Polycyclic Aromatic Hydrocarbons in Aquati Experiments (Faculty of Science and Forestry/Department of Biology University of Eastern Finland, Finland, 2013).
Wayland, M. et al. Levels of polycyclic aromatic hydrocarbons and dibenzothiophenes in wetland sediments and aquatic insects in the oil sands area of Northeastern Alberta, Canada. Environ. Monit. Assess. 136(1), 167–182 (2008).
Kraus, J. M. et al. Metamorphosis alters contaminants and chemical tracers in insects: implications for food webs. Environ. Sci. Technol. 48(18), 10957–10965 (2014).
Stoks, R. & Córdoba-Aguilar, A. Evolutionary ecology of Odonata: a complex life cycle perspective. Annu. Rev. Entomol. 57(1), 249–265 (2012).
Lu, X., Reible, D. D. & Fleeger, J. W. Bioavailability of polycyclic aromatic hydrocarbons in field-contaminated Anacostia River (Washington, DC) sediment. Environ. Toxicol. Chem. 25(11), 2869–2874 (2006).
Thomann, R. V. & Komlos, J. Model of biota-sediment accumulation factor for polycyclic aromatic hydrocarbons. Environ. Toxicol. Chem. 18(5), 1060–1068 (1999).
Lu, X. et al. Bioavailability of desorption-resistant phenanthrene to the oligochaete Ilyodrilus templetoni. Environ. Toxicol. Chem. 22(1), 153–160 (2003).
Brion, D. & Pelletier, É. Modelling PAHs adsorption and sequestration in freshwater and marine sediments. Chemosphere 61(6), 867–876 (2005).
Shilla, D. J. Distribution and behaviour of polycyclic aromatic hydrocarbons in subtropical river estuary, Okinawa, Japan. Chem. Ecol. 32(5), 472–491 (2016).
Bilotta, G. S. & Brazier, R. E. Understanding the influence of suspended solids on water quality and aquatic biota. Water Res. 42(12), 2849–2861 (2008).
Zhang, X. et al. Bioavailability of pyrene associated with suspended sediment of different grain sizes to daphnia magna as investigated by passive dosing devices. Environ. Sci. Technol. 49(16), 10127–10135 (2015).
Nielsen, K. et al. Particle phase distribution of polycyclic aromatic hydrocarbons in stormwater—using humic acid and iron nano-sized colloids as test particles. Sci. Total Environ. 532, 103–111 (2015).
Corbet, P. S. Dragonflies: Behaviour and Ecology of Odonata (Harley Books, Colchester, 1999).
Boxall, A. B. A. & Maltby, L. The effects of motorway runoff on freshwater ecosystems: 3. Toxicant confirmation. Arch. Environ. Contam. Toxicol. 33(1), 9–16 (1997).
Carls, M. G. et al. Fish embryos are damaged by dissolved PAHs, not oil particles. Aquat. Toxicol. 88(2), 121–127 (2008).
Moermond, C. T. A., Zwolsman, J. J. G. & Koelmans, A. A. Black carbon and ecological factors affect in situ biota to sediment accumulation factors for hydrophobic organic compounds in flood Plain Lakes. Environ. Sci. Technol. 39(9), 3101–3109 (2005).
Thomann, R. V., Connolly, J. P. & Parkerton, T. F. An equilibrium model of organic chemical accumulation in aquatic food webs with sediment interaction. Environ. Toxicol. Chem. 11(5), 615–629 (1992).
Burkhard, L. P. Factors influencing the design of bioaccumulation factor and biota-sediment accumulation factor field studies. Environ. Toxicol. Chem. 22(2), 351–360 (2003).
Meland, S.G., Tânia; Petersen, Karina; Håll, Johnny; Lund, Espen; Kringstad, Alfhild; Grung, Merete, DNA damage in dragonfly nymphs (Odonata, Anisoptera) living in highway sedimentation ponds. 2019: Manuscript submitted for publication.
Thorsen, W. A., Cope, W. G. & Shea, D. Bioavailability of PAHs: effects of soot carbon and PAH source. Environ. Sci. Technol. 38(7), 2029–2037 (2004).
Brooks, S. and S. Cham, Module 8: Advanced Identification of Dragonflies and Damselflies (Odonata): Larvae and Exuviae in The Identification of Freshwater Invertebrates to Species Level: a Distance-learning Course. 2009, Freshwater Biological Association. p. 23.
Goretti, E. et al. Larval development of Aeshna cyanea (Müller, 1764) (Odonata: Aeshnidae) in Central Italy. Hydrobiologia 457(1), 149–154 (2001).
Shrivastava, A. & Gupta, V. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2(1), 21–25 (2011).
Burkhard, L., Estimation of Biota Sediment Accumulation Factor (BSAF) from Paired Observations of Chemical Concentrations in Biota and Sediment. (U.S. Environmental Protection Agency, Ecological Risk Assessment Support Center, Cincinnati, 2009).
Wood, M., Beresford, N. & Copplestone, D. Limit of detection values in data analysis: do they matter?. Radioprotection 46(6), 85–90 (2011).
Acknowledgements
This work was supported and partly funded by the Danish, Norwegian, and Swedish Road Administration through their NordFoU R&D programme Reducing Highway Runoff Pollution (REHIRUP, Grant number 604133, https://www.nordfou.org). In addition, the work was partly funded by NIVA’s basic funding from the Research Council of Norway (Grant number 160016).
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and development of this study. V.G. and S.M. collected the samples. V.G. and M.G. performed the chemical analyses. V.G. was responsible for data management and statistical analysis, and the article’s first draft. All authors revised and gave the final approval for publication of this article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Girardin, V., Grung, M. & Meland, S. Polycyclic aromatic hydrocarbons: bioaccumulation in dragonfly nymphs (Anisoptera), and determination of alkylated forms in sediment for an improved environmental assessment. Sci Rep 10, 10958 (2020). https://doi.org/10.1038/s41598-020-67355-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-67355-1
- Springer Nature Limited
This article is cited by
-
Stormwater ponds serve as variable quality habitat for diverse taxa
Wetlands Ecology and Management (2024)
-
Multibiomarker responses to polycyclic aromatic hydrocarbons and microplastics in thumbprint emperor Lethrinus harak from a South Pacific locally managed marine area
Scientific Reports (2021)