Abstract
We aimed to characterise the response of locally advanced basal cell carcinoma (BCC) to systemic treatment with Vismodegib, a Hedgehog pathway inhibitor, by changes in the expression levels of Hedgehog pathway genes. Data were collected prospectively on 12 patients treated systemically for locally advanced BCC. Biopsy samples taken on admission and after treatment cessation were analysed pathologically and with the NanoString nCounter system to quantify the expression of 40 Hedgehog signaling pathway genes. Findings were compared before and after treatment, between complete and partial responders, and with localised BCC samples from 22 patients. Sixteen Hedgehog pathway genes changed significantly from before to after treatment. GAS1 was the only gene with a significantly different expression at baseline between complete responders (6 patients) and partial responders (4 patients) to Vismodegib (P = 0.014). GAS, GLIS2 and PRKACG1 showed different expression before treatment between the locally advanced and localised BCCs. The baseline expression level of GAS1 appears to be predictive of the response of locally advanced BCC to systemic Vismodegib treatment. A change in expression of many Hedgehog pathway genes, albeit expected by the known activity of Vismodegib, may nevertheless serve as an indicator of the response potential of the tumour.
Similar content being viewed by others
Introduction
Basal cell carcinoma (BCC) is the most common cancer in the world, with a lifetime risk of 20% and an incidence that has grown continuously over recent decades1,2. BCC accounts for 80% of all nonmelanoma skin cancers. It occurs with greatest frequency in Caucasian and elderly populations1,2. The tumours are generally slow-growing, rarely fatal (mortality rate, less than 0.1%), and seldom metastasise (up to 0.5% of all cases)1,2. For localised BCCs, surgical treatment has a 5-year cure rate of more than 95%3,4. However, for the minority of BCCs that are locally advanced (laBCC) or metastatic (metBCC), conventional treatment methods are inadequate, and systemic therapies are considered the appropriate approach2.
The Hedgehog (Hh) signal transduction pathway is one of the key regulators of cell proliferation and differentiation during embryogenesis and plays a major role in ensuring proper embryonic development5,6. In adults, the Hh pathway is normally inactive except in stem and skin cells and hair follicles. However, studies have shown that aberrant uncontrolled activation of the Hh pathway is maintained in 95% of sporadic BCCs in addition to several other malignancies7.
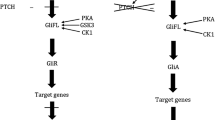
The Hh pathway is activated when the Hh ligand binds to and inhibits the Patched 1 (PTCH1) receptor, an inhibitor of the G-protein-coupled smoothed (SMO) transmembrane receptor. As a result, the activated SMO then inhibits the negative regulator Suppressor of Fused protein which binds glioma associated (GLI) transcription factors in the cytoplasm. The GLI transcription factors are then left free to enter the cell nucleus and promote cell division and tumourigenesis (Fig. 1A)5,6,8. Approximately 90% of sporadic BCCs demonstrate mutations in the PTCH1 gene, often associated with loss of heterozygosity; most of the remaining 10% show a gain-of-function SMO gene mutation. Both these mutations cause uncontrolled proliferation of skin basal cells9,10.
Vismodegib (Erivedge® Capsule, Genentech Inc., South San Francisco, CA, USA) is a first-generation synthetic small-molecule SMO receptor inhibitor. In 2012, the ERIVANCE study reported a 30% response rate to Vismodegib for metBCC and a 43% response rate for laBCC11. Prompted by these findings, the United States Food and Drug Administration approved the use of oral Vismodegib for the systemic biologic treatment of adults with laBCC or metBCC. The aim of the present study was to investigate the effect of Vismodegib on the expression levels of Hh pathway genes in laBCC and to search for potential predictors of tumour response.
Results
Twelve patients with laBCC and 22 patients with localised BCC were recruited for the study. The patient characteristics and clinical data are summarised in Table 1. Four patients in the laBCC group were excluded from the post-treatment analysis for the following reasons: receipt of a reduced dose of Vismodegib (n = 1); treatment with radiation prior to biopsy study (n = 1); tissue quality was too poor for evaluation (n = 1); or the biopsy sample was taken 6 months after cessation of treatment, when BCC recurred (n = 1).
Comparison of the laBCC tumours before systemic treatment with localised BCC tumours yielded a significant differential expression of three genes of the Hh pathway: GAS1, GLI similar 2 (GLIS2), and protein kinase CAMP-activated catalytic subunit gamma (PRKACG) (P < 0.05; Table 2). A heatmap demonstrating the similarity of the groups in the expression of all Hh pathway genes except these three is shown in Fig. 1B.
On comparison of the levels of expression of the Hh pathway genes before and after Vismodegib treatment in the laBCC group, a significant qualitative change was found in 16 genes (P < 0.05), as shown by the heatmap in Fig. 1C. They included several pivotal genes from the GLI, PTCH, and WNT families and SMO (Table 3). The patients (n = 11, with the exclusion of the patient given a reduced dose) were then divided by the endpoint of treatment according to the RECIST guidelines. Six patients had a complete response, 4 patients had a partial response, and one patient failed to respond. The latter patient was excluded from the analysis for statistical purposes. Molecular analysis revealed that the mean level of expression of GAS1 was significantly lower in the patients with a complete response compared to the patients with a partial response: 1874.83 ± 905.26 vs. 3901 ± 420.05, respectively (P = 0.014). This was the only Hh pathway gene showing a significantly different expression between these subgroups. There was no difference between the subgroups in the expression of GLI1 (1958.73 ± 1052.86 vs. 1753.17 ± 1539.18, respectively, P = 0.842).
Immunostaining to GAS1 on 4 localized BCC samples was compared to one normal skin sample in an attempt to show the feasibility of the staining. However, the BCC samples showed decreased staining compared to the normal skin, which precluded us from performing further staining on the complete and partial responders due so small amount of tissue remaining.
The localised BCCs were also compared by site: head and neck (n = 13), where sun exposure over time is substantial, and trunk (n = 9), where sun exposure is limited. A significant difference in three Hh pathway genes was noted between these subgroups: GLI3 (2207.11 ± 693.83 vs. 2853.74 ± 739.30, respectively, P = 0.049), protein kinase CAMP-dependent type 1 regulatory subunit beta (PRKAR1B; 2642.98 ± 1476.28 vs. 1444.93 ± 743.84, respectively P = 0.037), and wingless/integrated 2 (WNT2; 173.54 ± 141.20 vs. 39.75 ± 31.82, respectively P = 0.012).
Discussion
This study presents a comprehensive analysis of the response of Hh signalling pathway genes to treatment with Vismodegib in patients with laBCC. We found that before treatment, laBCCs were characterised by a significantly higher level of expression of three Hh pathway genes compared to localised BCCs. After systemic treatment of laBCC with Vismodegib, the expression of 16 Hh pathway genes changed from baseline. Division of the laBCC group by partial or complete response to treatment showed that a single gene, GAS1, was predictive of the tumour response.
NanoString nCounter analysis successfully revealed a significant decrease in the expression of many of the Hh pathway genes located downstream to the Vismodegib-inhibited SMO receptor. The ability of the NanoString nCounter system to quantify a large amount of mRNA simultaneously makes it a potential tool for determining tumour response to treatment and for identifying genes with consistent overexpression in resistant tumours that might be targeted for second-line therapy.
Further analysis to identify which of the genes could have predictive value in terms of tumour response to systemic treatment yielded only one, GAS1, which had a significantly higher level of expression in tumours with a partial response than in those with a complete response. The GAS1 protein modulates Hh pathway signalling together with two other cell-surface proteins, CAM-related/downregulated by oncogenes (CDO) and Brother of CAM-related/downregulated by oncogenes. GAS1 is the only one of the three that exists solely in vertebrates12,13. These 3 proteins function as coreceptors for the Hh ligand. They interact with the PTCH1 protein on the cell surface, increase its affinity for the ligand and thus enhance the Hh pathway14,15. Therefore, they potentially contribute to the excessive activation of the pathway, and several authors have already suggested their possible role in tumourigenesis15,16.
As Vismodegib inhibits the Hh pathway downstream to the activity of GAS1, its effect would not be expected to be influenced by the higher expression of GAS1. It is possible that the overexpression of GAS1 potentiates the pathway enough to overcome Vismodegib inhibition by releasing more SMO from PTCH1. Another possible explanation is that GAS1 has some effect, even if relatively mild, on SMO as well.
Patched 2 (PTCH2) is a second patched member, structurally similar to PTCH1, whose role as a tumour suppressor and response to Hh ligand have yet to be entirely understood. Several groups provided proof for at least some activity as a tumor suppressor and response to the Hh ligand, similarly to PTCH117,18. It is plausible that GAS1 also interacts with PTCH2 as a coreceptor. Thus, when GAS1 is overexpressed, PTCH2 has increased affinity to Hh ligand that inhibits its activity as a SMO inhibitor. This additional activation of SMO receptors (together with the mutated PTCH1 or SMO genes) could improve the tumour’s ability to resist some of the inhibition caused by Vismodegib, explaining the high GAS1 levels in the partially responsive laBCCs.
Interestingly, GAS1 was recently found to play a protective role against tumourigenesis and metastasis in colon and gastric cancers16,19,20. Its ability to suppress the tumour was attributed to its participation in the negative regulation of aerobic glycolysis, an essential requirement for tumour growth and spread19,20,21. In our study, the higher pretreatment GAS1 levels in the partially responding laBCCs may suggest that these tumours were more aggressive and therefore able to spread even with these GAS1 levels, making them relatively resistant to the systemic treatment.
GAS1 was one of only three Hh pathway genes that were differentially expressed in laBCCs and localised BCCs. The overexpression of GAS1 in laBCC may correspond to its higher level of expression in the partially treatment-responsive than in the completely responsive tumours. The importance of the other two genes, GLIS2 and PRAKCG, in this context has yet to be explored. The GLIS2 protein is a member of a subfamily of transcription factors that have a great impact on different physiological processes, including cancer. In contrast to GLIS1 and GLIS3, which are considered to be solely transcriptional activators, GLIS2 is also a repressor. However, there are no available data on its specific role in the Hh pathway or in BCC22. PRAKCG is part of the protein kinase C family which regulates cell differentiation and proliferation and inhibits apoptosis. Specifically, it was recently associated with the pathogenesis of several diseases and tumours, including colon and breast cancer. Its possible role in the Hh pathway or in BCC has not been investigated23,24.
Unfortunately, our attempt to use immunohistochemical staining to show overexpression of GAS1 in the partially vs. complete responsive laBCC was unsuccessful. We attempted to show the feasibility of the staining by comparing the expression of GAS1 in localised BCC vs. normal skin prior to using the remaining laBCC tissue for this staining. However, the results showed decreased staining in the BCC samples comparing to normal skin. This discrepancy between the immunohistochemical staining and the mRNA overexpression could be attributed to the fact that not all mRNA are translated to proteins. We suggest future studies using in situ hybridization staining to localise and determined the actual mRNA levels in the cells.
GLI1 expression is considered the most important indicator of the Hh pathway activity. There are three GLI transcription factors (GLI1-3): GLI1 is a full-length transcriptional activator, and GLI2 and GLI3 regulate the activity of GLI15,8. Atwood et al.7 recently compared the levels of GLI1 mRNA between normal skin, Vismodegib-sensitive and Vismodegib-resistant BCCs. Levels were slightly elevated in the sensitive tumours and high in the resistant tumours, suggesting the presence of genetic alterations that maintained the elevated output of the Hh pathway genes despite treatment. In the present study, there was no difference in the pretreatment levels of GLI1 between the tumours with a partial or complete final response or between localised BCCs and laBCCs. The high pretreatment levels may be explained by an upregulation of the Hh pathway in all BCCs. We speculate that during treatment, there is a drop in levels of GLI1, as an end product of the Hh pathway, unless the tumour successfully maintains excessive activity of the Hh pathway via bypass pathways or adjunctive mutations that the treatment cannot overcome.
A previous study investigated gene levels in BCCs compared to body surface areas exposed to sun25 and found that the BCCs had an overexpression of WNT2 and PRKAR1B together with an underexpression of GLI3. The Wnt pathway, similar to the Hh pathway, plays a pivotal role in embryonic development25, and overexpression of WNT has been identified in several types of cancers, causing proliferation, migration, and invasion of the tumour cells. The affected cancers included human melanoma and squamous cell carcinoma of the head and neck, both of which, like BCC, are related to sun exposure26,27,28,29. Further research should be directed at determining whether exposure to the sun is a direct cause of damage to these genes.
The main limitation of this study is the small sample size, a direct result of the relatively infrequent use of systemic treatment for BCCs. A large multicenter study is warranted. In situ hybridization staining should be used to confirm the findings and localizing the mRNA in future studies.
In summary, this study is the first to identify GAS1 baseline levels as a possible marker for the response of laBCCs to Vismodegib. This could lay the ground for future research of the role of GAS1 in the Hh pathway in general and in the resistance of the tumour to biologic treatment with Vismodegib. GAS1 might also serve as a target for future treatments.
Methods
Study design and patients
A prospective study was conducted at a tertiary medical center that serves as a national center for the systemic treatment of laBCC and metBCC. The study adhered to the tenets of the Declaration of Helsinki and was approved by the local institutional review board of Rabin Medical Center. The cohort included all patients with laBCC who were treated with oral Vismodegib between January 2015 and September 2016 and signed an informed consent to participate in the study. Exclusion criteria were previous tumour treatments with other modalities.
The patients were evaluated clinically by a dermatologist (G.T.) and a plastic surgeon (G.B.Y., D.A.) before and after cessation of treatment. The response to treatment was determined according to the RECIST guidelines30. Biopsy samples were taken for histological study and to molecularly quantify the expression of the different Hh pathway genes at both time points. The findings were compared from before to after treatment, between complete and partial responders, by tumour site and with samples of localised BCC taken from patients prior to tumour excision.
Molecular analysis procedure
Biopsy samples were fixed in formalin and embedded in paraffin, and 8–10 µl of mRNA were extracted at a concentration of 100–150 ng/µl using the RNeasy FFPE Kit (QIAGEN, Germany). The molecular analysis was performed by an external company using the NanoString nCounter system (Agentek Ltd., Israel) which directly quantifies gene expression with high precision and sensitivity. The system is capable of counting hundreds of unique mRNA transcripts in a single reaction without the need for reverse transcription or amplification31. For the present study, the expression levels of 40 genes in the Hh pathway were analysed in each sample from the patients and controls.
Tissue processing for immunohistochemistry
Four samples of BCC and one sample of normal skin were fixed in 4% paraformaldehyde, processed routinely and embedded in paraffin. Formalin fixed paraffin-embedded sample sections (3 μm) were mounted on superfrost slides. Hematoxylin and eosin staining was used for histological evaluation under light microscope. Sequential sections were used for GAS-1 staining.
GAS1 immunohistochemistry
The immunostains were performed on an automated stainer (Benchmark Ultra; Ventana Systems, Phoenix, AZ). Primary antibody incubation time was 28 minutes after antigen retrieval in a Tris based buffer (20 minutes at 95–100 °C). Polyclonal rabbit anti-human GAS1 antibody (17903–1-AP, Proteintech) (diluted 1:50) was used. The detection reaction used the ultraVIEW DAB detection kit (760–500 Ventana Systems, Phoenix, AZ), according to manufacturer-recommended protocol. Hematoxylin counterstain was used for color development.
Statistical analysis
Data for continuous variables are presented as mean and standard deviation and for nominal parameters as number. Unpaired Student’s t-test was used to compare gene expression levels in laBCC samples before and after treatment, between complete and partial response, with the localised BCC and between sites of localised BCC. A P value of < 0.05 was considered significant. All statistical analyses were performed using SPSS for Windows (SPSS, Inc. Chicago, IL, USA).
Data availability
All data generated or analysed during this study are included in this published article.
References
Hoorens, I., Vossaert, K., Ongenae, K. & Brochez, L. Is early detection of basal cell carcinoma worthwhile? Systematic review based on the WHO criteria for screening. Br J Dermatol. 174, 1258–1265 (2016).
Lanoue, J. & Goldenberg, G. Basal cell carcinoma: a comprehensive review of existing and emerging nonsurgical therapies. J Clin Aesthet Dermatol. 9, 26–36 (2016).
Chren, M. M. et al. Tumor recurrence 5 years after treatment of cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 133, 1188–1196 (2013).
Silverman, M. K., Kopf, A. W., Bart, R. S., Grin, C. M. & Levenstein, M. S. Recurrence rates of treated basal cell carcinomas. Part 3: Surgical excision. J Dermatol Surg Oncol. 18, 471–476 (1992).
Erdem, G. U., Sendur, M. A., Ozdemir, N. Y., Yazici, O. & Zengin, N. A comprehensive review of the role of the Hedgehog pathway and vismodegib in the management of basal cell carcinoma. Curr Med Res Opin. 31, 743–756 (2015).
Silapunt, S., Chen, L. & Migden, M. R. Hedgehog pathway inhibition in advanced basal cell carcinoma: latest evidence and clinical usefulness. Ther Adv Med Oncol. 8, 375–382 (2016).
Atwood, S. X. et al. Smoothened variants explain the majority of drug resistance in basal cell carcinoma. Cancer Cell. 27, 342–353 (2015).
Rimkus, T. K., Carpenter, R. L., Qasem, S., Chan, M. & Low, H. W. Targeting the sonic Hedgehog signaling pathway: review of Smoothened and GLI inhibitors. Cancers (Basel). 8, pii: E22 (2016).
Epstein, E. H. Basal cell carcinomas: attack of the hedgehog. Nat Rev Cancer. 8, 743–754 (2008).
Xie, J. et al. Activating smoothened mutations in sporadic basal-cell carcinoma. Nature. 391, 90–92 (1998).
Sekulic, A. et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 366, 2171–2179 (2012).
Allen, B. L. et al. Overlapping roles and collective requirement for the coreceptors GAS1, CDO, and BOC in SHH pathway function. Dev Cell. 20, 775–787 (2011).
McCabe, J. M. & Leahy, D. J. Smoothened goes molecular: new pieces in the Hedgehog signaling puzzle. J Biol Chem. 290, 3500–3507 (2015).
Veenstra, V. L. et al. Patched-2 functions to limit patched-1deficient skin cancer growth. Cell Oncol (Dordr). 41, 427–437 (2018).
Martinelli, D. C. & Fan, C. M. The role of Gas1 in embryonic development and its implications for human disease. Cell Cycle. 6, 2650–2655 (2007).
Segovia, J. & Zarco, N. Gas1 is a pleiotropic regulator of cellular functions: from embryonic development to molecular actions in cancer gene therapy. Mini Rev Med Chem. 14, 1139–1147 (2014).
Fleet, A. J. & Hamel, P. A. The protein-specific activities of the transmembrane modules of Ptch1and Ptch2 are determined by their adjacent protein domains. J Biol Chem. 293, 16583–16595 (2018).
Katoh, M. Genomic testing, tumor microenvironment and targeted therapy of Hedgehog-related human cancers. Clin Sci (Lond). 133, 953–970 (2019).
Wang, H. et al. Growth arrest-specific gene 1 is downregulated and inhibits tumor growth in gastric cancer. FEBS J. 279, 3652–3664 (2012).
Jiang, Z., Xu, Y. & Cai, S. Down-regulated GAS1 expression correlates with recurrence in stage II and III colorectal cancer. Hum Pathol. 42, 361–368 (2011).
Li, Q. et al. Gas1 inhibits metastatic and metabolic phenotypes in colorectal carcinoma. Mol Cancer Res. 14, 830–840 (2016).
Kim, Y. S., Kang, H. S. & Jetten, A. M. The Kruppel-like zinc finger protein Glis2 functions as a negative modulator of the Wnt/beta-catenin signaling pathway. FEBS Lett. 581, 858–864 (2007).
Zhang, Y. et al. Single-nucleotide polymorphisms of the PRKCG gene and osteosarcoma susceptibility. Tumour Biol. 35, 12671–12677 (2014).
Mazzoni, E., Adam, A., Bal de Kier Joffe, E. & Aquirre-Ghiso, J. A. Immortalized mammary epithelial cells overexpressing protein kinase C gamma acquire a malignant phenotype and become tumorigenic in vivo. Mol Cancer Res. 1, 776–787 (2003).
Kahn, M. Can we safely target the WNT pathway? Nat Rev Drug Discov. 13, 513–532 (2014).
Newton-Bishop, J. A. et al. Relationship between sun exposure and melanoma risk for tumours in different body sites in a large case-control study in a temperate climate. Eur J Cancer. 47, 732–741 (2011).
Kovacs, D. et al. The role of Wnt/beta-catenin signaling pathway in melanoma epithelial-to-mesenchymal-like switching: evidences from patients-derived cell lines. Oncotarget. 7, 43295–43314 (2016).
Webster, M. R., Kugel, C. H. III & Weeraratna, A. T. The Wnts of change: how Wnts regulate phenotype switching in melanoma. Biochim Biophys Acta. 1856, 244–251 (2015).
Aminuddin, A. & Ng, P. Y. Promising druggable target in head and neck squamous cell carcinoma: Wnt signaling. Front Pharmacol. 7, 244 (2016).
Nishino, M. et al. New Response Evaluation Criteria in Solid Tumors (RECIST) guidelines for advanced non-small cell lung cancer: comparison with original RECIST and impact on assessment of tumor response to targeted therapy. AJR Am J Roentgenol. 195, W221–W228 (2010).
Geiss, G. K. et al. Direct multiplexed measurement of gene expression with color-coded probe pairs. Nat Biotechnol. 26, 317–325 (2008).
Acknowledgements
This study was partially supported by the Zanvyl and Isabelle Krieger Fund, Baltimore, Maryland, USA, and Roche Holding AG, Basel, Switzerland. The funding organizations had no role in the design or conduct of this research. Partially presented at the Israel Ophthalmology Society (ILOS) meeting, Tel Aviv, June 2018 and ISOPT, Tel Aviv, March 2018.
Author information
Authors and Affiliations
Contributions
Conception and design: A. Sternfeld, S. Rosenwasser-Weiss, G. Ben-Yehuda, M.F.-Gohas, I. Yassur, G. Tauber, A. Olshinka, Y. Vardizer, D. Ad El and N. Goldenberg-Cohen. Development of methodology: S. Rosenwasser-Weiss, G. Ben-Yehuda, H. Kreizman Shefer, J. Bejar, M.F.-Gohas, G.Tauber, Y. Vardizer, D. Ad El and N. Goldenberg-Cohen. Acquisition of data: S. Rosenwasser-Weiss, G. Ben-Yehuda, M.F.-Gohas, I. Yassur, G. Tauber, A. Olshinka, Y. Vardizer, D. and Ad El. Analysis and interpretation: A. Sternfeld, S. Rosenwasser-Weiss, G. Ben-Yehuda, M.F.-Gohas, A. Olshinka, H. Kreizman Shefer, N. and Goldenberg-Cohen. Writing, review, and/or revision of the manuscript: A. Sternfeld, S. Rosenwasser-Weiss, G. Ben-Yehuda, H. Kreizman Shefer, N. and Goldenberg-Cohen.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sternfeld, A., Rosenwasser-Weiss, S., Ben-Yehuda, G. et al. Gene-Related Response of Basal Cell Carcinoma to Biologic Treatment with Vismodegib. Sci Rep 10, 1244 (2020). https://doi.org/10.1038/s41598-020-58117-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-58117-0
- Springer Nature Limited