Abstract
The aphid Schlechtendalia chinensis(Bell) induces horned galls on their primary host Rhus chinensis(Mill). These galls serve as closed habitats to support thousands of aphids per gall. Ecological parameters inside a gall are unknown. In this study, we showed that the microclimate inside galls was reltively stable, with nearly 100% humidity and 30–50 lux light regardless of outside environmental conditions. Gall-residing aphids produce waste gas and honeydew. A gall contained 26 organic volatiles inside with acetic acid as the largest component. Honeydew is rich in sugars and may provide nutrients for microbial growth. However, no evidence for pathogenic microorganisms was found inside a gall. The acidic environment in a gall may curb microbial growth. On the secondary host, the moss Plagiomnium maximoviczii (Lindb.) T. J. Kop., the microclimate is unstable and humidity fluctuated at 45~100%, while light ranged from 150 to 500 lux on different environmental conditions. Aphid alternated in two different habitats, the gall generation increased from a single fundatrix to thousands of aphids, however, survival rate of the moss generation is less 3%. A comparison of the environmental traits between gall and moss revealed that a stable habitat with dark and moist is advantageous for aphid reproduction.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
The horned sumac gall, found on the host plant Rhus chinensis (Mill), is induced by an aphid Schlechtendalia chinensis (Bell) and is the result of abnormal leaf wing rachis tissue growth. The aphid S. chinensis has a complex life cycle and exhibits cyclical parthenogenesis and gamogenesis by ovoviviparity on alternative hosts between tree and moss. During the life cycle of S. chinensis, aphids live alternately between its primary host tree, R. chinensis and secondary host moss, Plagiomnium maximoviczii (Lindb) T. J. Kop. Aphids migrate from galls to moss in October and remain there until March. From October to March, the aphids reside on the inner layer of soft moss where they reproduce one generation via parthenogenesis. The following spring in early April, the winged aphids generate when aphids turn into four instars, the winged aphid migrate from its winter host, the moss, to its primary host R. chinensis. Once aphids have reached the tree host, they deposit male and female sexuales in slits of branches. After mating, a female produces a single apterous fundatrix that feeds on the rachis wing and induces a closed gall, where fundatrix develop an adult aphid and reproduces three generations via parthenogenesis in it from May to October. At the end of October, the winged aphids migrate from broken galls to moss plant nearby under tree shade (Fig. 1)1,2,3. Rhus trees are distributed in most areas of China. However, the moss P. maximoviczii can only live in environments with high shade and moisture. Therefore, in nature, the horned sumac galling aphids are primarily distributed in regions around 25°~35°N latitude in China, where both Rhus trees and moss are present.
Although the aphid S. chinensis can live on both Rhus gall and moss hosts, the microenvironments of the two host species differ considerably, resulting in unique ecological and physiological challenges associated with each host for the aphid to adapt. While the environment on moss has been studied4, very little is known about the microenvironment inside the gall. Some researchers suggested that closed galls could provide a moderate effect on temperature buffering5,6,7. However, how to environmental change affect microclimate inside gall and inside layer of moss is still sealed. The gall is regarded as a structure that protects gall-inducing organisms from adverse environments, particularly desiccation8,9,10,11,12. However, dry environments in Australia do not result in more galls than in cooler, wetter environments13. Unlike some galling aphids induced gall that is for escaped from dry environment, horned gall by S. chinensis is only found environments with high moisture in China, specifically in regions with rainfall exceeding 1200 mm/year, relative humidity (RH) >80%. In spring, aphid S.chinensis migrate from its moss host to its tree host, which coincides with the start of the rainy season, gall generation occurred in rain season, whereas the fall migration from gall on Rhus trees to mosses also coincides with the beginning of dry season, moss generation lived in dry season. Galling aphids distributed in different regions could have their distinct migration behavior and ecological strategy to adapt adverse environment. A better understanding on the micro-ecological parameters in closed galls and the factors that drive aphid migration in autumn and spring will help to explain ecological adaptability of this aphid.
The gall of S. chinensis is a closed space in which thousands of individuals are housed during the later stages of aphid development14. Once the population density is high, the production of carbon dioxide and other waste gases levels will increase inside the gall, thus, raising the question as to how gas exchange with the exterior is regulated and how this affects the development of the aphid population. Moreover, gall-residing aphids produce substantial amounts of honeydew and at least one study shows that this is solved by absorption via the inner gall wall15. It is unclear, however, whether residual honeydew in the gall interior is still problematic, as it could provide a substrate for pathogenic microorganismal growth. Honeydew is a sugary liquid that may contaminate the colony both through physical exposure to a sticky substance as well as providing a breeding ground for pathogens16,17,18. However, despite high population densities within the gall we neither found evidence for microbial contamination on the inner gall surface nor transmission of pathogens among aphids. The apparent absence of disease and microbial growth suggested that defense mechanisms must have evolved to avoid or minimize disease vector transmission.
The objective of this study is to examine the dynamic microenvironmental changes that occur in aphid-produced galls and in living habitats on moss plants as well the consequences of these changes. Thus, we measured humidity, temperature, light intensity, concentration of volatiles in closed gall and acid concentration in the gall tissue. We also analysed other differences in environments associated with both host plants. This report discusses the parameters we observed between inside galls and in the layers of moss plants that have likely affected to aphid population growth. We also discuss the ecological reasons why aphids switch host plants.
Results
Structure of gall and volatile organic compounds inside gall
Horned gall grew in clusters on branches of host tree and a horned gall is a closed space (Fig. 2A,B). On the gall wall, stomata are distributed in a dense array of spines on the external surface of galls (Fig. 2C). We found stoma density on the surface of gall was only about one-third of that found on leaves of the host tree (Fig. 2D), gas exchange inside and outside of gall could be impeded. Thus, the respiratory activity of large aphid populations could generate and accumulate substantial amount of gas metabolites. In this study, 26 volatile organic compounds were identified in addition to high CO2 levels, these volatiles include carboxylic acids, alkanes, olefins, alcohols, ethers, aldehydes, ketones and nitriles (Table 1). Among these 26 volatiles, acetic acid was the dominant compound, accounting for more than one third of all volatiles. Given the high level of acetic acid, the pH condition inside galls should be low. Indeed, pH of ground gall tissues was only 4.97 ± 0.02 (n = 10). There were also some crevices and pores on the interior gall surface (Fig. 2E), possibly important for the removal of honeydew. Although there were thousands of aphids excreting honeydew inside a gall, we dissected many galls and found that the interior of a gall was actually quite clean, with no sign of microbial contamination (Fig. 2F,H).
Physical parameter of horned gall and aphid excreted honeydew. (A) Galls grow on tree. (B) A single horned gall. (C) Stomata on outside surface of gall. (D) Stomata comparison between gall and leaf (error bars denote SD, n = 30). (E) Internal surface of gall. (F) Aphids living inside a gall. (G) An aphid is excreting honeydew in gall. (H) Clean interior wall surface of a gall.
Temperature and humidity
Galls are thought to provide physical barriers that protect galling insects from physical and biological hazards. A closed gall also produces a unique ecological environment for galling insects to live. Our data revealed that the temperature and humidity of the gall interior were regular difference from the conditions outside. When temperature was between 27~33 °C on sunny days, the interior temperature of the gall was 2~3 °C (1.58 ± 1.045, t = −7.73, P > 0.01) below the outside temperature (Fig. 3A). In contrast, when temperatures were below 17.5 °C on cloudy or rainy days, temperatures inside the galls were 0.1~0.5 °C (0.16 ± 0.11 on clouds day; t = 3.61, P > 0.01 and 0.33 ± 0.10 on rainy day, t = 7.05, P > 0.01) higher than the outside temperatures (Fig. 3C,E). The interior humidity of the gall remained almost constant at 100%, regardless of the environmental conditions (Fig. 3B,D,F). These data indicate that the gall structure serves as a buffer temperature fluctuation in both directions and suggests that the gall wall is an effective barrier to prevent evaporation. Similar to the gall interior, the temperatures inside the layer of moss also changed to a lesser degree compared with those above the moss layer. When temperature of the atmosphere was over 23 °C, the temperatures inside the moss layer registered 0.1~2.3 °C (1.22 ± 0.56, t = −6.0, P > 0.01) less, while at outside temperatures lower than 23 °C the temperatures inside moss layer were 0.1~1.0 °C (0.58 ± 0.30, t = 8.13, P > 0.01) higher, suggesting that interior layer of the moss plants can also serves as also slightly buffers temperatures (Fig. 3G), but less effective compared with galls. The humidity inside the layers of moss plants is more than 70% on different weather conditions, humidity inside layer of moss is 70~99% when environmental humidity ranged from 45% to 90% on sunny days, and humidity inside layer of moss is almost 99.9% when environmental humidity ranged from 80% to 99% on cloudy days (Fig. 3H). Indicated that moss has better moisture retention as gall.
Temperature and relative humidity between environments, inside galls and in layers of moss plants(Error bars denote SD, n = 5). (A) Temperatures inside and outside galls on sunny day. (B) Relative humidity inside and outside galls in sunny day. (C) Temperatures inside and outside galls under overcast day. (D) Relative humidity between inside and outside galls under overcast day. (E) Temperatures inside and outside galls in rainy day. (F) Relative humidity inside and outside galls in rainy day. (G) Temperatures inside and above layers of moss plants. (H) Relative humidity inside and above layers of moss plants on sunlight and overcast days.
Light conditions inside galls and moss layers
The average light intensity outside the gall was 4932.5 ± 74.25 Lux on sunny days, but the average light intensity was 35.60 ± 7.78 Lux inside galls, the inside illuminance at less than 1% of this amount. On cloudy days, the average light intensity outside the gall was 572.47 ± 26.87 Lux. In comparison, the average light intensity was 40.8 ± 6.0 Lux inside the gall, representing 7% of that observed outside gall intensity. Despite much lower light intensity, light conditions remained relatively stable (35~40 Lux) inside galls under different weather conditions. On the other hand, the light intensity inside the layers of moss reached 150~500 Lux on cloudy and sunny days, which was much higher than those of inside galls (Table 2).
Aphid population dynamics in galls and on moss plants
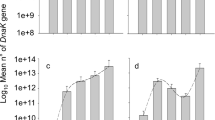
There are significant differences in the fertility and population size of aphids that residing in galls and on moss plants. Inside a gall, a fundatrix generated a huge volume aphids via parthenogenesis in the subsequent three generations, which span seven months of life in the gall interior. On the secondary host moss, aphids resided for five months and produced one generation via parthenogenesis, however, the population size was dramatically reduced with survival rates being less than 3% (2.86 ± 1.10) in the open field and about 17% (17.34 ± 9.15) under the black shade cloth cage conditions (Fig. 4A). The ecological conditions under the black shade cloth cage and the open field site were somehow similar with the exception of higher humidity and lower light intensity, and overall more stable conditions under the cage, which are likely the reason for the higher aphid fecundity in the cage. Under natural conditions, aphids on moss plants live in the so-called dry season, whereas aphids in galls live in the rainy season (Fig. 4B).
Population dynamic and environmental conditions between gall and moss. (A) Population dynamic inside gall and on moss (error bars denote SD, n = 30). (B) The weather conditions of gall generation and moss generation. (C) An aphid is secreting wax on moss (white arrows) and wax covered aphid on moss (black arrows).
Discussion
The aphid S. chinensis has a complex life cycle that exposes the insect to different environments between the galls on sumac tree and its winter host moss. Our study revealed that aphid habitats on both Rhus trees and moss plants could provide a high humidity environment with low light conditions. Humidity and light intensity appeared to important environmental factors that influence aphid populations. The differences between these two hosts were higher humidity and lower light intensity in closed gall, and high humidity and low light intensity conditions in galls remained relatively stable under different weather conditions. On the other hand, humidity and light intensity fluctuated quite significantly on moss plants.
The microenvironment of high humidity and low light intensity inside a gall may provide benefits to the residing aphids. High humidity inside a gall could be primarily due to the respiration of large aphid populations in a closed system. Plant stomata are an important channel for exchange of gas and water vapor between gall interior and exterior environment. Stomate density on the gall surface is only one-third of that found on leaves of host trees, indicating that gall wall is less efficient for gas exchange and may have contributed to maintaining high moisture (100%) and gas accumulation inside the gall. In the a more open moss environment, humidity inside layer of moss fluctuated depending on the other environmental conditions but humidity is over 70%.
Overwhelming majority of aphids need light in their development and reproduction. For aphids living within a gall, most direct sunlight is blocked by the gall wall. Nevertheless, the gall interior has low but stable light conditions, with 35~40 Lux during day time under different weather conditions. On the other hand, the moss host grows in the shade, and illuminance in the moss varied from 150 to 500 lux during overcast or sunny days, thus exceeding the illuminance of the gall interior by at least 5~10-fold. Consistent with this, aphids secret a waxes substance while inhabiting on the moss host, presumably to cover their bodies with a wax layer that reduces exposure to light and water evaporation of aphid body (Fig. 4C). Galls play a role in temperature buffering: The gall wall can attenuate temperature changes inside the gall when environmental temperatures fluctuate. These observations confirm the idea that closed galls provide a moderate effect on temperature buffering5,6,7. A similar ability to buffer temperatures was also observed for the moss plants. However, temperature appears to not be of critical importance as moisture and light intensity for aphid development and reproduction, because the effect of temperature buffering is only limited in both galls and moss layers. In fact, the aphid S. chinesis is distributed between 25°~35°N latitude, and populations can survive in a wide range of temperatures.
There are crevices and pores throughout inner layer of a gall, which likely improve liquid wastes (such as water and honeydew) removal. Honeydew droplets are rich in sugars and may result in microbial growth if not removed. Kutsukake et al. (2012) indicated that honeydew in closed gall absorbed via the inner gall wall15. However, the residuum of honeydew is always on the interior surface of gall, which could breed microorganism. Despite high aphid population densities within the galls we neither found evidence for microbial contamination on the inner gall surface nor transmission of pathogens among aphids. An interesting possibility is that atmospheric conditions inside the gall contribute to maintaining a quasi-sterile environment to counter these hazards. Twenty-six volatile organic compounds were detected in galls, the main gas component among volatiles is acetic acid, which accounts for more than one third of all volatiles. Acetic acid and other volatile organic compounds such as β-phellandrene and octanoic acid may inhibit the growth of microorganisms19,20,21,22. In addition, high levels of tannic acid (>70%) were also found in gall tissue23,24, which may also help to curb microorganism growth25,26. Thus, it is possible that the combination of these microenvironmental parameters allow S. chinensis to thrive in a closed and crowded enclosure without any obvious microorganism contamination.
Many insects modify their environments directly, rather than merely choosing available sites that are already favorable, they constructed feeding or resting shelters such as galls5. The gall provides a shelter to aphids to avoid an adverse environment and protection against natural enemies. More importantly, this structure also provides a suitable microenvironment for growth and development of the aphid. In comparison, layers of moss plants are more labile to exposure to environmental extremes. The advantage of a gall can also be seen from aphid population expansion. Aphids reproduce three generations in a gall, resulting in thousands of offspring form from a single aphid fundatrix via parthenogenesis. In contrast, tens of thousands of aphids migrate from an individual gall to moss layers, where they reproduce for only one generation with less 3% of the initial population that can survive2 but bigger population sizes of aphids in moss layers under a in black cage. These results indicated that the low reproductive and survival rates of aphids in moss layers are likely due to high variation in microenvironments.
For most aphids, the evolution of complex life cycles may be driven by selection to segregate ecological resources such as food and refuge27. The complementary host growth hypothesis27,28,29,30,31,32,33 could explain why aphids migrate from tree to moss hosts because trees without leaves in Fall produce less energy and nutrients during winter, resulting in nutritional loss. Besides food resource, host alternation could escape from natural enemies and adverse environments, particularly in arid micro-habitats5,9,10,11,12. However, S. chinensis resides on the moss host during the dry season from October to March, while gall growth occurred in the rainy season from May to September, indicating that in this case the gall may not be a strategy to escape from desiccation since S. chinensis can undergo the dry season in a relatively unprotected habitat (on moss). Before the rainy season starts, aphids complete the spring migration from the moss to the tree host where they hide under tree bark until leaves sprout. The fact that aphids prefer living under tree barks to staying on moss plants indicates some reasons other than nutrients drive the spring migration. This suggests that one possible reason could be rainfall, as heavy rain will submerge the moss and reduce aphid survival. Additionally, there is period of time with higher light duration and intensity from May to September (Fig. 4B), which could be another reason for spring migration because the aphid prefers to a lucifuge condition to live. In the life cycle of S. chinensis on alternative two hosts, galls represent the principal habitat31, but the moss serves as a transitional host because R.chinensis is a deciduous tree, and as such loses leaves in winter, which would compromise nutrient availability for the gall and aphids. A comparison of the environmental traits between gall and moss suggested that aphids prefer a dark and moist habitat, while the gall provides additional safety from environmental hazards.
Materials and Methods
Gall collection
Field measurements were done on the fresh galls collected from the experimental site (N 28°06′E 104°22′, elevation 820 m) located in the natural distribution of gall, Yanjin county, Yunnan province, Southwest China. Climate condition: Annual average temperature is 16~18 °C; relative humidity is more than 80%; rainfall 1200~1300 mm. The 8-years old Rhus trees were used for gall production. The moss P. maximoviczii was planted on 3 cm fine grained soil under tree shade.
Comparative examination on stomata between galls and leaf
To analyze and compare stoma condition between gall and leaf, we counted stoma numbers on the surface between gall walls and leaves by using a Scanning Electron Microscope (Hitachi TM3000, Hitachi Co, Japan). Thirty datasets were obtained randomly on stoma average numbers for both gall walls and leaves respectively.
Measurements of volatile organic compounds
Measurements of organic volatiles were conducted at 10:00 a.m. on a sunny day in late August, During the measurement, a syringe with to a micro-pump was inserted into a gall grow on Rhus tree, volatiles from gall were pumped to the chamber by a micro-pump and collected by the sampling tube with adsorbents Tenax TA (0.2 g, 60–80 mesh, Perkin-Elmer) for 2 hours. Sampling tubes were sealed, stored 4 °C, and then analyzed by an ATD-GC-MS system (Automated thermal desorber-gas chromatography/mass spectrometry)34. All samples were analyzed in triplicates.
pH measurement
The pH of fresh gall tissue was measured with a pH meter (HI2002-02, HANNA ITALY) after grinding. Ten fresh galls were used to pH measurements.
Measurements of temperature and humidity in the gall and layers of moss
Gall experiments were performed in field in late August (when galls were fully developed). A minuscule hole (with a diameter 3~4 mm) was drilled through the gall and into a gall wall to allow the insertion of a miniature probe. The hole was sealed with wax after the probe was inserted into the gall, when parameters inside gall become stable, temperature and humidity were recorded by auto-thermohygrometers (Testo 635–2, Germany). Temperatures and humidity were recorded in parallel inside and outside a gall (5 cm away from the gall) during a range of conditions, including direct sunshine, overcast conditions and rain. Measurements were recorded every 10 minutes for 24 hours. Five samples were collected from five trees (one gall per tree). Measurements of microenvironmental parameters for the moss occurred in January. A miniature probe was inserted under a layer of the moss and another probe above the layer, and temperature and humidity were recorded every 10 minutes for 24 hours. Temperature and humidity of every hour were described at averaged value of six time recorded in one hour and plotted graph.
Measurements of light penetrating into a gall
A hole was drilled into the gall wall as described in the previous section to allow the insertion of a miniature luminometer probe (Testo 150, Germany). The hole was then sealed with wax. Light inside gall was recorded 3 minutes after the light intensity became stable, The illuminance inside the gall during different weather conditions and determined light intensity outside the gall in parallel were recorded. Thirty galls were selected and measured randomly. A miniature luminometer probe was also inserted into a layer of moss to detect illuminance inside moss plants and another probe above the layer to determined light intensity above the moss in parallel. Thirty data were obtained randomly in 150 m2 moss.
Recording dynamics of aphid population in the galls and on the moss plants
Prior to aphid migration to the moss plant, thirty gall samples were randomly collected, Aphids in individual galls were counted by opening the gall. To determine aphid population sizes on moss plants, we set up two experimental sites, one in black shade cloth cage (5 m × 4 m × 3 m) and one in the field. We planted moss P. maximoviczii on fine grains soil, in black shade cloth cage, illuminance allowed us to keep in the 100~200 lux range and humidity at 80%~90%. At the open field site, we measured illuminance at 100~500 lux and humidity at 50%~80% for host mosses that grew in the tree shade. At the beginning of autumn migration, 100 migrated aphids bred on an area of 50 cm2 moss. Prior to spring migration, we counted aphids again in the same area. Data from thirty samples were respectively obtained in both black tent and open field.
Weather data
Weather data (temperature, rainfall and light) was obtained from the weather bureau of Yanjing county (horned sumac gall natural distribution area). Average temperatures, and rainfalls, and light intensities for the latest 30 years mean value was used for the analysis. The weather station is 15 kilometers away from experiment site.
Statistical analysis
Statistical analysis was conducted using the IBM SPSS Statistics 19 software. T-test was conducted for temperature comparisons between inside gall, moss and environment.
References
Lai, Y. Q. et al. Biological basis for rearing horned gall aphid, Schlechtendalia chinensis. Forest Research (1992).
Lai, Y. Q. et al. Biological characteristics of horned gall aphid and changes in quantity during overwintering. Forest Research (1990).
Lai, Y. Q. Preliminary studies on the biology of horned gall aphid in the gall and the growth of the gall. Forest Research (1988).
Qiu, M. & Zhao, Z. Study on Growth Characteristics of A Winter Host (Plagiomnium maximoviczii) of Horned Gall Aphid (Schlechtendalia chinensis). Chinese Journal of Ecology (1999).
Danks, H. V. Modification of Adverse Conditions by Insects. Oikos 99(1), 10–24 (2002).
Layne, J. R. Jr. Microclimate variability and the eurythermic nature of goldenrod gall fly (Eurosta solidaginis) larvae (Diptera: Tephritidae). Canadian Journal of Zoology 69(3), 614–617 (1991).
Layne, J. R. Jr. Winter microclimate of goldenrod spherical galls and its effects on the gall inhabitant Eurosta solidaginis (Diptera: Tephritidae. Journal of Thermal Biology 18(3), 125–130 (1993).
Cornell, H. V. The Secondary Chemistry and Complex Morphology of Galls Formed by the Cynipinae (Hymenoptera): Why and How? American Midland Naturalist 110(2), 225–234 (1983).
Barnes, K. J. Biology of Insect-Induced Galls. Annals of the Entomological Society of America 86(1), 122–123 (1993).
Fay, P. A., Hartnett, D. C. & Knapp, A. K. Increased photosynthesis and water potentials inSilphium integrifoliumgalled by cynipid wasps. Oecologia 93(1), 114–120 (1993).
Fernandes, G. W. & Price, P. W. The adaptive significance of insect gall distribution: survivorship of species in xeric and mesic habitats. Oecologia 90(1), 14–20 (1992).
Crespi, B. J. & Worobey, M. Comparative Analysis of Gall Morphology in Australian Gall Thrips: The Evolution of Extended Phenotypes. Evolution 52(6), 1686–1696 (1998).
Blanche, K. R. Diversity of insect-induced galls along a temperature- rainfall gradient in the tropical savannah region of the Northern Territory, Australia. Austral Ecology 25(4), 311–318 (2010).
Shao, S. X., Yang, Z. X. & Chen, X. M. Gall development and clone dynamics of the galling aphid Schlechtendalia chinensis (Hemiptera: Pemphigidae). Journal of Economic Entomology 106(4), 1628–1637 (2013).
Kutsukake, M. et al. An insect-induced novel plant phenotype for sustaining social life in a closed system. Nature Communications 3(6), 1187 (2012).
Stadler, B. & Müller, T. Aphid Honeydew and Its Effect on the Phyllosphere Microflora of Picea abies (L.) Karst. Oecologia 108, 771–776 (1996).
Way, J. M. Mutualism Between Ants and Honeydew-Producing Homoptera. Annu.rev.entomol 8(1), 307–344 (1963).
Perez, J. L. et al. Fungal Phyllosphere Communities are Altered by Indirect Interactions Among Trophic Levels. Microbial Ecology 57(4), 766–774 (2009).
Conková, E., Para, L. & Kocisová, A. Inhibition of growth of microscopic fungi with organic acids. Veterinární medicína 38(12), 723–727 (1993).
Kang, H. C., Park, Y. H. & Go, S. J. Growth inhibition of a phytopathogenic fungus, Colletotrichum species by acetic acid. Microbiological Research 158, 321–326 (2003).
Plumridge, A. et al. The Weak Acid Preservative Sorbic Acid Inhibits Conidial Germination and Mycelial Growth of Aspergillus niger through Intracellular Acidification. Applied & Environmental Microbiology 70(6), 3506–3511 (2004).
Peláez, A. M. L. et al. Inhibitory activity of lactic and acetic acid on Aspergillus flavus growth for food preservation. Food Control 24(1-2), 0–183 (2012).
Hang, C. et al. Molecular mechanisms of tannin accumulation in Rhus galls and genes involved in plant-insect interactions. Scientific Reports 8, 1:9841 (2018).
Wang, H. et al. Gibberellic acid is selectively downregulated in response to aphid-induced gall formation. Acta Physiologiae Plantarum 38(9), 214 (2016).
Brownlee, H. E. et al. Anti-fungal effects of cocoa tannin on the witches’ broom pathogen Crinipellis perniciosa. Physiological & Molecular Plant Pathology 36(1), 39–48 (1990).
Achmad, et al. Effects of Tannin to Control Leaf Blight Disease on Toona sureni Merr. Caused by Two Isolates of Rhizoctonia sp. Plant Pathology Journal (2015).
Hardy, N. B., Peterson, D. A. & Von Dohlen, C. D. The evolution of life cycle complexity in aphids: Ecological optimization or historical constraint? Evolution 69(6), 1423–1432 (2015).
Kennedy, J. S. & Booth, C. O. Host alternation in Aphis fabae Scop. I Feeding preferences and fecundity in relation to age and kind of leaves. Annals of Applied Biology 38(1), 25–64 (2008).
Dixon, A. F. G. The life-cycle and host preferences of the bird cherry-oat aphid, Rhopalosiphum padi L., and their bearing on the theories of host alternation in aphids. Annals of Applied Biology 68(2), 135–147 (1971).
Whitham, T. G. Habitat Selection by Pemphigus Aphids in Response to Response Limitation and Competition. Ecology 59, 1164–1176 (1978).
Moran, N. A. The Evolution of Aphid Life Cycles. Annual Review of Entomology 37(37), 321–348 (1992).
Moran, N. A. The Evolution of Host-Plant Alternation in Aphids: Evidence for Specialization as a Dead End. American Naturalist 132(5), 681–706 (1988).
Moran, N. A. Adaptation and Constraint in the Complex Life Cycles of Animals. Annual Review of Ecology and Systematics. 25(1), 573–600 (2003).
Hua, Z. Study on Volatile Components of Butterfly Nectar Plants and Host Plants. Asian Journal of Chemistry 25(14), 7861–7863 (2013).
Acknowledgements
This study was funded by the National Natural Science Foundation of China (Grant Nos U1402263, 31872305), the National Key R&D Program of China (2018YFD0600403), the grant for Innovative Team of “Insect Molecular Ecology and Evolution” of Yunnan Province, the Program for Innovative Research Team (in Science and Technology) in University of Yunnan Province (IRTSTYN) and the Ph.D. Research Initiation Project (Grant No. 01102/111910).
Author information
Authors and Affiliations
Contributions
C.W. wrote a first draft. C.W., P.L. and S.X.S performed the experiments of microclimate and population dynamics. X.M.C. designed the experiments and wrote the manuscript. J.L., Q.L., Z.X.Y. and H.C. detected organic volatile compounds inside gall and taken Scanning Electron Microscope. King-Jone K. revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, C., Liu, P., Chen, X. et al. Microenvironmental analysis of two alternating hosts and their impact on the ecological adaptation of the horned sumac gall aphid Schlechtendalia chinensis (Hemiptera, Pemphiginae). Sci Rep 10, 435 (2020). https://doi.org/10.1038/s41598-019-57138-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-57138-8
- Springer Nature Limited