Abstract
Cape honeybee, Apis mellifera capensis, workers can be social parasites and host colonies can defend themselves by rejection of such workers. Using the pseudo-clonal obligate parasitic lineage of A. m. capensis and wild-type A. m. capensis workers, which are facultative parasites, we show that host colonies significantly increase their defence behaviour towards social parasites upon secondary exposure. Most obligate and facultative social parasites were rejected before they could even produce significant amounts of the queen-like mandibular gland pheromone secretion or activate their ovaries. This suggests that other signals, like cuticular hydrocarbons, could be used by host colonies to identify potential invaders. Honeybee colonies seem to be able to utilise these potential cues, learn from their initial exposure to hive intruders and enable them to improve their defensive behaviour during subsequent infestations, resulting in increased removal rates of parasites.
Similar content being viewed by others
Introduction
Cape honeybee, Apis mellifera capensis Eschscholtz, workers can be social parasites in colonies of their own and other subspecies, such as the neighbouring one, Apis mellifera scutellata Lepeletier1,2,3,4,5,6,7,8. After successful invasion, socially parasitic workers often develop a pseudoqueen phenotype9 including the activation of ovaries and the production of a queen-like pheromonal bouquet10. These workers parthenogenetically produce female offspring via thelytoky11. In the course of infestation, the host queen is lost7. The colonies are then gradually taken over and eventually die7,9.
Since 1990, socially parasitic A. m. capensis workers have invaded A. m. scutellata colonies on a large scale, resulting in the loss of thousands of colonies per year6,7,12. As there is high genetic variance for reproductive dominance in A. m. capensis9,13 selection for the most dominant socially parasitic worker genotype is favoured within the A. m. scutellata host population. In fact, the pseudo-clonal offspring of a single parasitic worker11,14,15 have caused infestations in the north-eastern parts of South Africa and is now covering an area of approximately 275.000 km2,14. All parasitic workers in the range of A. m. scutellata bear the genetic signature of a clone founded by a single ancestral worker genotype11,14,15.
Despite this “capensis calamity”12, A. m. scutellata colonies have some resistance, because host workers can show rejection behaviour, e.g. attack and eject parasitic workers9. Previous studies on parasite infection have shown that honeybee colonies may eject small hive beetle invaders more quickly after a second exposure16. This might also be the case for socially parasitic workers and could be adaptive given high chances of multiple infections. Moreover, identification might be specific to certain types of parasites even within a subspecies. To test if honeybee workers have the ability to identify specific, even closely related, parasites, we took advantage of the pseudo-clonal socially parasitic worker lineage of the Cape honeybee, A. m. capensis7,11,17. If honeybee colonies are able to recognize this lineage specifically, we would expect them to remove such parasites more quickly compared to other honeybee lineages and subspecies.
Results
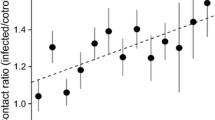
The survival analysis revealed that during both infections the parasitic pseudo-clones were identified and removed significantly faster than A. m. scutellata control (Fig. 1; pseudo-clone vs. scutellata control: Infestation 1 Test statistics (TS) = 4.74 p < 0.00001; Infestation 2 TS = 7.58 p < 0.00001). Only during the second infestation were the parasitic pseudo-clones identified and removed significantly faster than A. m. capensis non-clonal social parasites (Fig. 1; pseudo-clone vs. A. m. capensis non-clonal: Infestation 1 TS = 2.23 n.s.; Infestation 2 TS = 2.7 p < 0.0068). Furthermore, A. m. scutellata control workers were removed significantly more slowly by the host colonies than A. m. capensis non-clonal social parasites (scutellata vs. non-clonal parasites: TS > 2.32, p < 0.02 for infection 1 and 2). For each individual test group, individuals of the same group were removed at a faster rate from the test colonies during the second encounter as compared to the first infections (TS > 8.45, p < 0.00001, for all three pair wise comparisons). By comparing the increase in the average daily mortality from the first to the second round of infections it was revealed that the pseudo-clonal group increased from an average mortality of 19.7% to 39.4% (z = 4.31, p < 0.0001) during the second infestation, the A. m. scutellata control group increased from 13.8% average daily mortality to 21.3% (z = 1.66, n.s.) and the non-clonal parasitic group from 16.8% to 31.4% (z = 3.38, p < 0.001).
Proportion of control, obligate and facultative parasitic workers surviving per day after sequential infections. Comparison of the survival of the three different worker groups in each infection using Cox-Mantel tests. Mean and SE of the cumulative proportion of survival by day of the 1st and 2nd infection (36 h later) are shown. First letter, if different, indicates significant differences in survival between different worker groups within first infection. Second letter, if different, indicates significant differences in survival between different worker groups within second infection. Significant differences between the sequential infections within one worker group are indicated by asterisks.
Discussion
The data clearly show that both, the socially parasitic pseudo-clonal and the non-clonal social parasites A. m. capensis workers were rejected significantly faster upon secondary exposure.
Pheromonal competition governs reproductive dominance hierarchies among A. m. capensis workers18,19. The tested clonal parasitic worker lineage has probably been selected for rapid queen-like mandibular gland secretions20,21 as well as for its resistance against host queen pheromone signals19,22. The observed identification and rejection of socially parasitic workers during the first infection by the host workers is therefore most likely related to the faster reproductive development of the pseudo-clonal lineage23, similar to aggressive behaviour shown by non-developed workers towards workers with ongoing reproductive development in other subspecies24. Furthermore, internal anatomical differences like spermatheca size and numbers of ovarioles25 could result in differential volatile profiles. Pheromones could be another set of factors, in particular the pheromonal predisposition of A. m. capensis workers seen even under queenright conditions26 and the fact that the gland secretion of the parasitic lineage is significantly different to subordinate workers27. These differences in the gland and cuticular profile would allow the host workers to learn parasite specific cues, which allows them to identify and remove them in a subsequent infestation26,27.
Furthermore, the results show that during the second encounter the pseudo-clonal parasitic workers are rejected at a much faster rate by the A. m. scutellata host workers than all other tested groups. That pseudo-clonal parasitic workers are rejected faster could either be because of the genetically homogeneity of the lineage, which could make it is easier to learn its specific cues for the host or it is indicating a specifically directed behavioural response. More than 90% of the pseudo-clonal parasitic workers were rejected in less than 24 hours (Fig. 1) and the faster removal of the non-clonal social parasites suggests that it is rather a specifically directed behavioural response. This would mean that the faster the behavioural reaction, the stronger the resulting protection of the host colony as A. m. capensis workers need on average 6.5 days to become reproductively active23. Indeed, the callow parasitic workers did not remain long enough in the host colonies to activate their ovaries23, but signals from the tergal gland and cuticle could be used to discriminate between hosts and parasites19,26. Therefore, the parasitic workers could not establish themselves as reproductive parasites and can be excluded before they are able to gain reproductive dominance9,26 and before they are able to overthrow colony defences. In any case, the observed fast behavioural reaction of the host workers upon secondary exposure provides strong support to earlier findings for the ejection of small hive beetle parasites by honeybee colonies16. Taken these observations together, it appears as if honeybee colonies may be able to learn from their initial exposure to hive intruders and are able to improve their defensive behaviour, resulting in increased removal rates of parasites. The observation that the host colonies are able to evict social parasitic workers more swiftly during the second encounter could either suggest that the host colonies get conditioned or that honeybees have evolved a potential social immune memory to fight conspecific parasites. Both are not mutually exclusive and would result in the same advantageous behaviour of repelling potential social parasites.
Material and Methods
Study animals and experimental set up
Queenright, unrelated A. m. scutellata colonies (N = 4) were obtained from its endemic range (Pretoria, Gauteng Province, South Africa). All of them were set up in three-frame observation hives (∼3,000 bees). The middle frame in each hive contained brood and the top and bottom frames honey and pollen. Colonies were fed ad libitum with sugar water (1:1) and artificial beebread (honey/icing sugar/soya flour 1:2:2). On the same day of establishing the observation hives, frames with sealed worker brood were placed in an incubator until adult emergence28. Twenty-four hours later, we introduced three different groups of freshly emerged individually labelled test workers (N = 50 each group, <24 h old) into the four queenright test colonies:
-
1)
A. m. capensis social parasites with diverse genotypes (facultative parasitic lineage): offspring of a naturally mated queen from the endemic range of the Cape honeybee (Heidelberg, Western Cape).
-
2)
A. m. capensis social parasites with pseudo-clonal genotypes (obligate parasitic lineage): thelytokous offspring of the socially parasitic A. m. capensis worker lineage11,14,29 from a heavily infected queenless A. m. scutellata host colony (Pretoria, Gauteng30).
-
3)
A. m. scutellata host workers: offspring of a naturally mated queen from Pretoria (Gauteng) unrelated to the recipient colonies.
The introduction of all 150 workers took less than five minutes and freshly emerged workers were used because they are normally readily accepted31 as the colony odour in honeybees is strongly affected by environmental cues32. The experiments were conducted in 2004, all workers were labelled individually with Opalithplättchen (numbered plastic tags) and observation hives were screened for the presence of the introduced workers twice a day (13:00 day light and 18:00 red light conditions) following previously used methods33.
Procedure
All observation hives were infected at the same time and screened twice daily for the presence of labelled workers for 10 days and after the period, all test workers were carefully removed. The infections were repeated with new test workers 36 h thereafter using the same colonies and protocols.
Data analyses
The data were analysed using Cox-Mantel survival analysis. Pair-wise comparisons were performed between the different groups and the Bonferroni adjustment was applied. The data are displayed as the cumulative proportion surviving. For the Cox-Mantel analysis both the test statistic (TS) and p values are given. To evaluate if any of the groups received a stronger behavioural rejection during the second round of infection we calculated the overall average daily mortality and statistically compared the proportions. All statistical analyses were performed using Statistica©.
References
Onions, G. W. South African ‘fertile worker bees’. Agri Jour Union So Africa 3, 720–728 (1912).
Velthuis, H. H. W., Ruttner, F., Crewe, R. M. & Engels, W. Differentiation in Reproductive Physiology and Behaviour during the Development of laying worker honeybees in Social Insects: an evolutionary approach to castes and reproduction 231–243 (Springer Verlag, 1990).
Beekman, M., Wossler, T. C., Martin, S. & Ratnieks, F. L. W. Parasitic Cape honey bee workers (Apis mellifera capensis) are not given differential treatment by African guards (A. m. scutellata). Insectes Soc 49, 216–220 (2002).
Martin, S., Beekman, M., Wossler, T. C. & Ratnieks, F. L. W. Parasitic Cape honeybee workers, Apis mellifera capensis, evade policing. Nature 415, 163–165 (2002).
Pirk, C. W. W. et al. Reproductive Biology of the Cape Honeybee: A Critique of Beekman et al. J Hered 103, 612–614, https://doi.org/10.1093/jhered/ess007 (2012).
Härtel, S., Raassen, F. S., Neumann, P., Moritz, R. F. A. & Hepburn, H. R. Social parasitism by Cape honeybee workers in colonies of their own subspecies (Apis mellifera capensis Esch.). Insectes Soc 53, 183–193 (2006).
Neumann, P. & Hepburn, H. R. Behavioural basis for social parasitism of Cape honeybees (Apis mellifera capensis). Apidologie 33, 165–192, https://doi.org/10.1051/apido:2002008 (2002).
Beekman, M. & Oldroyd, B. P. When workers disunite: Intraspecific parasitism by eusocial bees. Annu Rev Entomol 53, 19–37, https://doi.org/10.1146/annurev.ento.53.103106.093515 (2008).
Neumann, P. & Moritz, R. F. A. The Cape honeybee phenomenon: the sympatric evolution of a social parasite in real time? Beh Ecol Sociobiol 52, 271–281, https://doi.org/10.1007/s00265-002-0518-7 (2002).
Crewe, R. M. & Velthuis, H. H. W. False queens: A consequence of mandibular gland signals in worker honeybees. Naturwissenschaften 67, 467–469 (1980).
Baudry, E. et al. Whole-Genome Scan in Thelytokous-Laying Workers of the Cape Honeybee (Apis mellifera capensis): Central Fusion, reduced Recombination Rates and Centromere Mapping Using Half-Tetrad Analysis. Genetics 167, (243–252 (2004).
Allsopp, M. H. & Crewe, R. M. The Cape bee as a Trojan horse rather than the hordes of Genghis Khan. Am Bee J 133, 121–123 (1993).
Jordan, L. A., Allsopp, M. H., Beekman, M., Wossler, T. C. & Oldroyd, B. P. Inheritance of Traits Associated with Reproductive Potential in Apis mellifera capensis and Apis mellifera scutellata Workers. J Hered 99, 376–381 (2008).
Neumann, P., Härtel, S., Kryger, P., Crewe, R. M. & Moritz, R. F. A. Reproductive division of labour and thelytoky result in sympatric barriers to gene flow in honeybees (Apis mellifera L.). J Evol Biol 24, 286–294, https://doi.org/10.1111/j.1420-9101.2010.02167.x (2011).
Aumer, D. et al. A single SNP turns a social honey bee (Apis mellifera) worker into a selfish parasite. Mol Biol Evol 36, 516–526, https://doi.org/10.1093/molbev/msy232 (2019).
Halcroft, M., Spooner-Hart, R. & Neumann, P. Behavioral defense strategies of the stingless bee, Austroplebeia australis, against the small hive beetle, Aethina tumida. Insectes Soc 58, 245–253, https://doi.org/10.1007/S00040-010-0142-X (2011).
Moritz, R. et al. Rare royal families in honeybees, Apis mellifera. Naturwissenschaften 92, 488–491, https://doi.org/10.1007/s00114-005-0025-6 (2005).
Moritz, R., Lattorff, H. & Crewe, R. Honeybee workers (Apis mellifera capensis) compete for producing queen-like pheromone signals. Proc R Soc Lond, B, Biol Sci 271, S98–S100 (2004).
Okosun, O. O., Pirk, C. W. W., Crewe, R. M. & Yusuf, A. A. Glandular sources of pheromones used to control host workers (Apis mellifera scutellata) by socially parasitic workers of Apis mellifera capensis. J Insect Physiol 102, 42–49, https://doi.org/10.1016/j.jinsphys.2017.09.001 (2017).
Dietemann, V., Neumann, P., Härtel, S., Pirk, C. W. W. & Crewe, R. M. Pheromonal dominance and the selection of a socially parasitic honeybee worker lineage (Apis mellifera capensis Esch.). J Evol Biol 20, 997–1007, https://doi.org/10.1111/j.1420-9101.2007.01303.x (2007).
Moritz, R. F. A., Pirk, C. W. W., Hepburn, H. & Neumann, P. Short-sighted evolution of virulence in parasitic honeybee workers (Apis mellifera capensis Esch.). Naturwissenschaften 95, 507–513 (2008).
Dietemann, V., Pflugfelder, J., Härtel, S., Neumann, P. & Crewe, R. M. Social parasitism by honeybee workers (Apis mellifera capensis Esch.): evidence for pheromonal resistance to host queens’ signals. Beh Ecol Sociobiol V60, 785–793 (2006).
Ruttner, F. & Hesse, B. Rassenspezifische Unterschiede in Ovarentwicklung und Eiablage von weisellosen Arbeiterinnen der Honigbiene Apis mellifera L. Apidologie 12, 159–183 (1981).
Visscher, P. K. & Dukas, R. Honey bees recognize development of nestmates’ovaries. Anim Behav 49, 542–544 (1995).
Phiancharoen, M., Pirk, C. W. W., Radloff, S. E. & Hepburn, R. Clinal nature of the frequencies of ovarioles and spermathecae in Cape worker honeybees, Apis mellifera capensis. Apidologie 41, 129–134 (2010).
Zheng, H.-Q. et al. Pheromonal predisposition to social parasitism in the honeybee Apis mellifera capensis. Behav Ecol 21, 1221–1226, https://doi.org/10.1093/beheco/arq131 (2010).
Okosun, O. O., Yusuf, A. A., Crewe, R. M. & Pirk, C. W. W. Effects of age and Reproductive Status on Tergal Gland Secretions in Queenless Honey bee Workers, Apis mellifera scutellata and A. m. capensis. J Chem Ecol, 1–8, https://doi.org/10.1007/s10886-015-0630-6 (2015).
Neumann, P., Radloff, S. E., Pirk, C. W. W. & Hepburn, H. R. The behaviour of drifted Cape honeybee workers (Apis mellifera capensis): predisposition for social parasitism? Apidologie 34, 585–590, https://doi.org/10.1051/apido:2003048 (2003).
Aumer, D., Mumoki, F. N., Pirk, C. W. W. & Moritz, R. F. A. The transcriptomic changes associated with the development of social parasitism in the honeybee Apis mellifera capensis. Sci Na 105, 22, https://doi.org/10.1007/s00114-018-1552-2 (2018).
Härtel, S. et al. Infestation levels of Apis mellifera scutellata swarms by socially parasitic Cape honeybee workers (Apis mellifera capensis). Apidologie 37, 462–470, https://doi.org/10.1051/apido:2006012 (2006).
Grout, R. A. The hive and the honey bee. (Dadant and Sons, 1946).
Bethe, A. Dürfen wir den Ameisen und Bienen psychische Qualitäten zuschreiben? Pflügers Arch Gesamte Physiol Menschen Tiere 70, 15–100 (1898).
Neumann, P., Pirk, C. W. W., Hepburn, H. R. & Radloff, S. E. A scientific note on the natural merger of two honeybee colonies (Apis mellifera capensis). Apidologie 32, 113–114, https://doi.org/10.1051/apido:2001116 (2001).
Acknowledgements
Appreciation is expressed to the late Randall Hepburn for stimulating discussions and for providing laboratory facilities. We like to thank Stephan Härtel, Manja Wendt, Frederike Raasen, Thomas Mürrle and Anton Schehle for technical support and we would like to extend our deepest gratitude to Cathy Bester for language editing and advice. The National Research Foundation and University of Pretoria provided finanical support.
Author information
Authors and Affiliations
Contributions
C.W.W.P. and P.N. designed the study, conducted the observations, collected the data, analysed and interpreted data, prepared the figures and wrote the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Neumann, P., Pirk, C.W.W. Increased response to sequential infections of honeybee, Apis mellifera scutellata, colonies by socially parasitic Cape honeybee, A. m. capensis, workers. Sci Rep 9, 7582 (2019). https://doi.org/10.1038/s41598-019-43920-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-43920-1
- Springer Nature Limited