Abstract
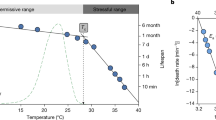
Recent studies suggest that animals are decreasing in size as a general response to global warming, for reasons that remain unclear. Here, by analysing ectotherm death time curves that take into consideration the intensity and duration of a thermal challenge, we show that heat tolerance varies predictably with size. Smaller animals can maintain higher body temperatures than larger ones during short periods, but cannot maintain higher body temperatures over long periods as their endurance declines more rapidly with time. Body size effects and adaptive variation in heat tolerance may have been obscured in the past by these unaccounted for temporal effects. With increasing size, thermal death occurs at relatively lower metabolic rates with respect to rest at a non-stressful temperature, which might partly explain the reported reductions in organism size with climate warming and shed light on the mechanisms that underlie scaling.



Similar content being viewed by others
Data availability
The datasets and R computer scripts associated with this article are open access available in DRYAD (https://doi.org/10.5061/dryad.zkh189380).
References
Smith, J. J., Hasiotis, S. T., Kraus, M. J. & Woody, D. T. Transient dwarfism of soil fauna during the Paleocene–Eocene thermal maximum. Proc. Natl Acad. Sci. USA 106, 17655–17660 (2009).
Sheridan, J. A. & Bickford, D. Shrinking body size as an ecological response to climate change. Nat. Clim. Change 1, 401–406 (2011).
Daufresne, M., Lengfellner, K. & Sommer, U. Global warming benefits the small in aquatic ecosystems. Proc. Natl Acad. Sci. USA 106, 12788–12793 (2009).
Gardner, J. L., Peters, A., Kearney, M. R., Joseph, L. & Heinsohn, R. Declining body size: a third universal response to warming? Trends Ecol. Evol. 26, 285–291 (2011).
Gillooly, J. F., Brown, J. H., West, G. B., Savage, V. M. & Charnov, E. L. Effects of size and temperature on metabolic rate. Science 293, 2248–2251 (2001).
Brown, J. H., Gillooly, J. F., Allen, A. P., Savage, V. M. & West, G. B. Toward a metabolic theory of ecology. Ecology 85, 1771–1789 (2004).
Martinez del Rio, C. & Karasov, W. H. Body size and temperature: why they matter. Nat. Educ. Knowl. 3, 10 (2010).
Araújo, M. B. et al. Heat freezes niche evolution. Ecol. Lett. 16, 1206–1219 (2013).
Klockmann, M., Günter, F. & Fischer, K. Heat resistance throughout ontogeny: body size constrains thermal tolerance. Glob. Change Biol. 23, 686–696 (2017).
Leiva, F. P., Calosi, P. & Verberk, W. C. Scaling of thermal tolerance with body mass and genome size in ectotherms: a comparison between water-and air-breathers. Philos. T. R. Soc. B. 374, 20190035 (2019).
Sinclair, B. J., Vernon, P., Klok, C. J. & Chown, S. L. Insects at low temperatures: an ecological perspective. Trends Ecol. Evol. 18, 257–262 (2003).
Rezende, E. L., Castañeda, L. E. & Santos, M. Tolerance landscapes in thermal ecology. Funct. Ecol. 28, 799–809 (2014).
Santos, M., Castañeda, L. E. & Rezende, E. L. Making sense of heat tolerance estimates in ectotherms: lessons from Drosophila. Funct. Ecol. 25, 1169–1180 (2011).
Rezende, E. L., Tejedo, M. & Santos, M. Estimating the adaptive potential of critical thermal limits: methodological problems and evolutionary implications. Funct. Ecol. 25, 111–121 (2011).
Strang, T. J. K. A review of published temperatures for the control of pest insects in museums. Coll. Forum 8, 41–67 (1992).
Sunday, J. M., Bates, A. E. & Dulvy, N. K. Global analysis of thermal tolerance and latitude in ectotherms. Proc. Natl Acad. Sci. USA 278, 1823–1830 (2010).
Hoffmann, A. A., Chown, S. L. & Clusella–Trullas, S. Upper thermal limits in terrestrial ectotherms: how constrained are they? Funct. Ecol. 27, 934–949 (2013).
May, R. M. How many species are there on earth? Science 241, 1441–1449 (1988).
Sunday, J. M. et al. Thermal–safety margins and the necessity of thermoregulatory behavior across latitude and elevation. Proc. Natl Acad. Sci. USA 111, 5610–5615 (2014).
Pinsky, M. L., Eikeset, A. M., McCauley, D. J., Payne, J. L. & Sunday, J. M. Greater vulnerability to warming of marine versus terrestrial ectotherms. Nature 569, 108–111 (2019).
Kearney, M. R., Gillingham, P. K., Bramer, I., Duffy, J. P. & Maclean, I. M. A method for computing hourly, historical, terrain‐corrected microclimate anywhere on Earth. Methods Ecol. Evol. 11, 38–43 (2020).
Rezende, E. L., Bozinovic, F., Szilágyi, A. & Santos, M. Predicting temperature mortality and selection in natural Drosophila populations. Science 369, 1242–1245 (2020).
Glazier, D. S. A unifying explanation for diverse metabolic scaling in animals and plants. Biol. Rev. 85, 111–138 (2010).
Schmid, P. E., Tokeshi, M. & Schmid-Araya, J. M. Relation between population density and body size in stream communities. Science 289, 1557–1560 (2000).
Pörtner, H. O. & Knust, R. Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 315, 95–97 (2007).
Fan, Y. & van den Dool, H. A global monthly land surface air temperature analysis for 1948–present. J. Geophys. Res. Atmos. 113, 1–18 (2008).
Reynolds, R. W., Rayner, N. A., Smith, T. M., Stokes, D. C. & Wang, W. An improved in situ and satellite SST analysis for climate. J. Clim. 15, 1609–1625 (2002).
Crisp, D. J. Methods for the Study of Marine Benthos 2nd edn (eds Holme, N. A. & McIntyre, A. D) 284–366 (Blackwell, 1984).
Reiss, J. & Schmid‐Araya, J. M. Existing in plenty: abundance, biomass and diversity of ciliates and meiofauna in small streams. Freshw. Bol. 53, 652–668 (2008).
Burnham, K. P. & Anderson, D. R. Model Selection and Multimodel Inference: A Practical Information–Theoretic Approach (Springer, 2002).
Turkheimer, F. E., Hinz, R. & Cunningham, V. J. On the undecidability among kinetic models: from model selection to model averaging. J. Cereb. Blood Flow. Metab. 23, 490–498 (2003).
Acknowledgements
We thank M. Santos for discussions on the subject and insightful comments on a first draft. This work was partly funded by a ANID PIA/BASAL FB0002 grant and a FONDECYT grant no. 1170017 to E.L.R.
Author information
Authors and Affiliations
Contributions
I.P.-M. and E.L.R. designed the study, compiled and analysed the data. Both authors contributed to the final draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Climate Change thanks Félix Leiva and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Distribution of measured and standardized Tcrit and warming tolerance.
Supplementary Fig. 1 Distribution of (a) measured and standardized Tcrit and (b) warming tolerance (warming tolerance = Tcrit - Tmax where Tmax corresponds to the mean temperature of the warmest month). Note that the standardization procedure tends to decrease not only mean Tcrit and warming tolerance but also their variance. Importantly, extreme values decrease to a substantially degree, ultimately increasing the signal-to-noise ratio of subsequent analyses. Tcrit was standardized for a 24 h exposure after accounting for mass effects.
Extended Data Fig. 2 Bias estimation in measured versus standardized Tcrit.
Supplementary Fig. 2 Bias estimation in measured versus standardized Tcrit. (a) Reported critical temperature estimates Tcrit versus standardized values for a 24 h exposure after accounting for mass effects. (b) Difference between Tcrit before and affecter the standardization procedure, to obtain an estimate of bias, plotted against standardized Tcrit, which controls for confounding effects of variable exposure times and body size differences. Similar reported and standardized Tcrit fall on the dotted line in both panels.
Extended Data Fig. 3 Geographic variation in Tcrit and warming tolerance.
Supplementary Fig. 3 Geographic variation in Tcrit and warming tolerance. Association between critical temperature estimates Tcrit and warming tolerance versus latitude and maximum temperature Tmax, estimated as the mean temperature of the warmest month. Upper and lower panels show replicated analyses for (a,b) reported versus standardized Tcrit for a 24 h exposure against absolute latitude, (c,d) Tcrit against maximum temperature Tmax and (e, f) warming tolerance estimated as Tcrit - Tmax along a latitudinal gradient.
Extended Data Fig. 4 Number of measurements per species in the dataset.
Supplementary Figs. 4 Number of measurements per species in our dataset, showing that a few species in our analyses have been measured more than once (see main text).
Extended Data Fig. 5 Body size and time distribution in the analysed datasets.
Supplementary Figs. 5 Body size and time distribution in the two datasets employed in our analyses (n = 328 measurements for Dataset 1 and 309 for Dataset 2).
Supplementary information
Supplementary Information
Supplementary methods for heat tolerance and metabolic scaling comparisons, Tables 1–4, Figs. 1–5 and references.
Rights and permissions
About this article
Cite this article
Peralta-Maraver, I., Rezende, E.L. Heat tolerance in ectotherms scales predictably with body size. Nat. Clim. Chang. 11, 58–63 (2021). https://doi.org/10.1038/s41558-020-00938-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41558-020-00938-y
- Springer Nature Limited
This article is cited by
-
Warming underpins community turnover in temperate freshwater and terrestrial communities
Nature Communications (2024)
-
A comprehensive database of amphibian heat tolerance
Scientific Data (2022)
-
Anthropogenic disruptions to longstanding patterns of trophic-size structure in vertebrates
Nature Ecology & Evolution (2022)
-
Experimental manipulation of microbiota reduces host thermal tolerance and fitness under heat stress in a vertebrate ectotherm
Nature Ecology & Evolution (2022)
-
Body size shapes thermal stress
Nature Climate Change (2021)