Abstract
Backgrounds/Objectives
The threshold of hypertension was lowered from systolic blood pressure (SBP)/diastolic blood pressure (DBP) 140/90 mmHg to 130/80 mmHg by the 2017 American College of Cardiology/American Heart Association blood pressure (BP) guideline. Thus, we investigated the association between retinal vein occlusion (RVO) occurrence and early-stage hypertension.
Subjects/Methods
This retrospective cohort study used the JMDC Claims Database (JMDC Inc., Tokyo, Japan) between 2005 and 2020. Individuals undergoing health checkups who had data on BP and did not take antihypertensive medications were included. They were classified into four BP groups: normal BP (SBP < 120 mmHg and DBP < 80 mmHg), elevated BP (SBP 120–129 mmHg and DBP < 80 mmHg), stage 1 hypertension (SBP 130–139 mmHg or DBP 80–89 mmHg), and stage 2 hypertension (SBP ≥ 140 mmHg or DBP ≥ 90 mmHg). Date of RVO occurrence was defined as the first date of diagnosis. We estimated adjusted hazard ratios for RVO and central RVO using weighted Cox regression to adjust for potential confounders.
Results
A total of 2,703,264 individuals were eligible. During a mean follow-up of 1,091 days, 3,526 RVO and 828 central RVO events occurred. The adjusted hazard ratios (95% confidence intervals) were 1.37 (1.19–1.57), 1.95 (1.75–2.18), and 3.33 (2.95–3.76) for RVO and 1.44 (1.07–1.93), 2.17 (1.72–2.73), and 3.76 (2.91–4.86) for central RVO in the elevated BP, stage 1 hypertension, and stage 2 hypertension groups, respectively, compared with the normal BP group.
Conclusions
Even individuals with early-stage hypertension showed higher risks for RVO and central RVO than individuals with normal BP.
Similar content being viewed by others
Introduction
Retinal vein occlusion (RVO) is one of the most common causes of visual loss [1]. In a meta-analysis performed in 2010, the age- and sex-standardised prevalence of any RVO was 5.20/1,000 population (0.80/1,000 for central RVO) [2], corresponding to more than 40 million people when projected to the world population in 2021 [3]. Although the prognosis of RVO has improved since the appearance of intravitreal ranibizumab and aflibercept [4,5,6,7,8], these drugs are expensive and confer a burden on medical budgets [9]. Furthermore, the prognosis of central RVO remains limited compared with that of branch RVO [6]. Thus, it is important to prevent RVO before its onset.
One of the major and modifiable risk factors for RVO is hypertension [1, 2, 10,11,12,13]. The 2017 American College of Cardiology (ACC)/American Heart Association (AHA) blood pressure (BP) guideline defined elevated BP as systolic BP (SBP) 120–129 mmHg and diastolic BP (DBP) < 80 mmHg, and stage 1 hypertension as SBP 130–139 mmHg or DBP 80–89 mmHg [14]. The BP threshold for hypertension was lowered from SBP/DBP ≥ 140/90 mmHg to SBP/DBP ≥ 130/80 mmHg by these new definitions. Previous studies investigated the association between RVO and hypertension using the old definition of hypertension (SBP/DBP ≥ 140/90 mmHg) [11,12,13]. However, there is little data on whether elevated BP and stage 1 hypertension have specifically higher risks for RVO occurrence compared with normal BP. If such associations are shown, the validity of the 2017 ACC/AHA BP guideline would improve, and the information would be valuable for not only ophthalmologists but also internists.
Therefore, using a large administrative claims database in Japan, we aimed to determine the associations between RVO occurrence and hypertension groups defined by the ACC/AHA guideline, especially whether elevated BP and stage 1 hypertension have higher risks for RVO development than normal BP (SBP/DBP ≤ 120/80 mmHg). We also assessed the proportions of RVO events that would be potentially preventable if elevated BP and stage 1 hypertension were reduced to normal BP using population attributable fractions [15].
Subjects and methods
Data source
This was a retrospective cohort study using data from the JMDC Claims Database (JMDC Inc., Tokyo, Japan) from 2005 to 2020 [16]. The database collects health insurance claims data from multiple insurance associations and the cumulative number of subjects was approximately 7.3 million in April 2020 [17]. De-identified and individual-level data for both outpatients and inpatients are stored. A strength of the database is that we were able to perform patient-based tracking of visits and treatment flows even if the individual was transferred to another hospital during treatment. The information included was as follows: 1) unique identifier; 2) patient characteristics (age and sex); 3) diagnoses based on International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes and diagnoses more-finely classified by Japanese text codes; 4) procedures; 5) drugs dispensed based on the Anatomical Therapeutic Chemical Classification System; 6) period from start to end of insurance; and 7) workplace employee annual health checkup data. The checkup data included numerical data, such as body mass index, SBP, DBP, high-density lipoprotein-cholesterol, low-density lipoprotein-cholesterol, triglycerides, and fasting blood sugar, and self-reported health questionnaire data, such as medical history (cerebrovascular disease, cardiovascular disease, renal failure/dialysis), smoking status (current smoker, ex-/non-smoker), alcohol consumption (daily, sometimes, rarely), and medication use (for hypertension, diabetes mellitus, dyslipidaemia). Because the individuals included in the JMDC database are company employees and their families, the database contains only a small number of individuals aged > 65 years and no individuals aged > 75 years [16]. The present study was performed in accordance with the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of The University of Tokyo. The need for informed consent was waived because of the anonymous nature of the database.
Patient selection
First, we identified individuals who underwent workplace employee annual health checkups and had look-back periods of ≥1 year. This look-back period was set to exclude prevalent cases of RVO. We defined the date of the first health checkup as the index date for each individual. Next, we excluded the individuals with missing data on BP and those taking antihypertensive medications. Furthermore, we excluded those with a history of cardiovascular disease [18], cerebrovascular disease [12, 19], renal failure/dialysis [11, 20], and/or hypercoagulable state [21] at the index date, because these diseases have been shown to be associated with RVO and they can be regarded as distinct populations. Those with missing data on the history of these diseases were also excluded. We excluded individuals who had experienced RVO before the index date (prevalent cases). Finally, we excluded data concerning health checkups that were performed outside of an individual’s observation period. Follow-up of patients started at the index date and ended at the date of RVO occurrence or last date of data collection. The study design is shown in Supplementary Fig. 1.
Exposure
Exposure was defined as BP category at the index date based on the 2017 ACC/AHA BP guideline: normal BP (untreated SBP < 120 mmHg and untreated DBP < 80 mmHg), elevated BP (untreated SBP 120–129 mmHg and untreated DBP < 80 mmHg), stage 1 hypertension (untreated SBP 130–139 mmHg or untreated DBP 80–90 mmHg), and stage 2 hypertension (untreated SBP ≥ 140 mmHg or untreated DBP ≥ 90 mmHg). The Japanese Ministry of Health, Labour and Welfare recommends a specific protocol for obtaining BP measurements in the health checkup system using a standard sphygmomanometer or an automated device [22]. BP was measured on the right arm after participants had rested for 5 min in a seated position. The measurement was performed twice with an interval of ≥ 1 min, and the average value was used.
Outcomes
We defined RVO occurrence as the first date of RVO diagnosis. The ICD-10 code H348 was used to identify any RVO and the more finely-classified Japanese text codes that contain ‘central’ RVO were used to identify central RVO.
Covariates
We included the following covariates at the index date: sex [13], age [1, 2, 10], obesity [1, 23], diabetes mellitus [13, 24], dyslipidemia [13, 24], smoking status [10, 25], alcohol consumption [18, 26], and glaucoma [13, 27]. These covariates were selected because they have been shown to be associated with occurrence of RVO. Obesity was defined as body mass index ≥ 25 kg/m2 [2, 28]. Diabetes mellitus was defined as fasting glucose ≥ 126 mg/dL or use of glucose-lowering medications [28]. Dyslipidaemia was defined as low-density lipoprotein-cholesterol ≥ 140 mg/dL, high-density lipoprotein-cholesterol < 40 mg/dL, triglycerides ≥ 150 mg/dL, or use of lipid-lowering medications [28]. Glaucoma was defined one or more dispensation of any antiglaucoma drug (Anatomical Therapeutic Chemical Classification code S01E, excluding S01EB09) during a 1-year period before the index date.
Statistical analysis
For the complete-case analysis, we excluded individuals with missing data for any covariate. We compared the baseline characteristics between the four BP groups using absolute standardised differences [29]. An absolute standardised difference < 0.1 indicated that the covariates were well-balanced. We estimated the crude cumulative incidence of RVO over 7 years in the entire population using the Kaplan–Meier estimator. This period was chosen because at least 10% of the individuals should remain to avoid a misleading interpretation of cumulative incidence [30]. We estimated crude hazard ratios (HRs) for RVO development using Cox regression in which RVO occurrence was regressed on the BP groups. To further examine the trend, we performed Cox regression in which RVO occurrence was regressed on the BP groups regarded as continuous variables [31]. The assumption of proportional hazards was graphically checked by Schoenfeld residual plots [32]. We replaced the follow-up period of 0 days with 0.5 days (n = 272) because the period of 0 days was considered an illegal time interval in the R ‘survival’ package, which was used in the present study [33].
To minimise potential confounding, we used a matching weight method to balance the baseline characteristics between the groups [34]. The matching weights were based on propensity scores and were shown to be generalizable to multiple treatment groups, demonstrating improved performance over 1:1:1 propensity score matching [34]. To obtain the propensity scores, we constructed multiple binary logistic regression models in which the BP groups were regressed on sex, age, obesity, diabetes mellitus, dyslipidaemia, smoking, alcohol consumption, glaucoma, interaction of age and sex, and interaction of sex and obesity. Using the propensity scores, we calculated matching weights and weighted the BP groups. We checked the balance of the covariates between the weighted groups using standardised differences. We calculated the cumulative incidence of RVO over 7 years for each weighted group using the Kaplan–Meier estimator. We estimated adjusted HRs for RVO compared with the normal BP group using weighted Cox regression. To further examine the trend, we performed Cox regression in which RVO occurrence was regressed on the BP groups regarded as continuous variables. The assumption of proportional hazards was graphically checked by Schoenfeld residual plots. Similar analyses were performed separately for central RVO.
We then calculated the population attributable fraction of each BP group for RVO. Population attributable fraction is a widely used measure to assess the impact of exposures in populations [15]. In the present study, the population attributable fraction represented the RVO case reduction in the population that would occur if stage 1 hypertension was entirely replaced with normal BP. 95% confidence intervals (CIs) were estimated using the Bonferroni inequality method [35]. Similar analyses were performed separately for central RVO.
For the sensitivity analysis, we divided the entire population into two age groups (young [<40 years] and middle-aged [≥ 40 years]).
All analyses were conducted using R software version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria) with the WeightIt package (version 0.9.0) for calculation of weights. A significance level of 5% was used in all analyses.
Results
A flow chart of the patient selection is shown in Fig. 1. A total of 2,755,335 individuals were eligible. Data were missing for 50,847 (1.8%) individuals for alcohol consumption, 1774 (0.06%) for smoking status, 790 (0.03%) for obesity, 35 (<0.001%) for diabetes mellitus, and 12 (<0.001%) for dyslipidaemia. After removing these individuals with missing data for covariates, we finally included 2,703,262 individuals for the complete-case analysis.
*The index date was defined as the date of the health checkup. †Missing data was found in 50,847 (1.8%) individuals for alcohol consumption, 1774 (0.06%) for smoking, 790 (0.03%) for obesity, 35 (<0.001%) for diabetes mellitus, and 12 (<0.001%) for dyslipidaemia. Other covariates had no missing data. BP blood pressure.
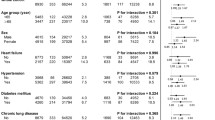
The patient characteristics before and after weighting by the matching weight method are shown in Table 1. Before weighting, individuals with elevated BP, stage 1 hypertension, and stage 2 hypertension were older and more likely to be male, have obesity, diabetes mellitus, and dyslipidaemia, be a current smoker, drink alcohol frequently, and have glaucoma than individuals with normal BP. After weighting, the absolute standardised differences were <0.1 (Supplementary Fig. 2).
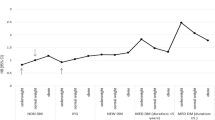
During the mean follow-up of 1,091 days (standard deviation, 925 days; range, 0–4773 days), 3,526 RVO events occurred. The crude cumulative incidence of RVO in the entire population was 0.31% over 7 years. The adjusted cumulative incidence of RVO over 7 years was lowest in the normal BP group (0.31%), followed by the elevated BP group (0.37%), stage 1 hypertension group (0.55%), and stage 2 hypertension group (0.89%) (Fig. 2). The crude HRs for branch RVO occurrence were 1.71 (95% CI, 1.53 to 1.92) for elevated BP, 3.42 (3.14 to 3.71) for stage 1 hypertension, and 6.68 (6.05 to 7.38) for stage 2 hypertension, compared with normal BP (Table 2). The adjusted HRs for branch RVO occurrence were 1.37 (1.19 to 1.57) for elevated BP, 1.95 (1.75 to 2.18) for stage 1 hypertension, and 3.33 (2.95 to 3.76) for stage 2 hypertension, compared with normal BP (Table 2). The P for trend was <0.001. The population attributable fractions for RVO for elevated BP, stage 1 hypertension, and stage 2 hypertension were 3.5% (1.6% to 5.4%), 20.6% (17.0% to 24.2%), and 13.8% (12.0% to 15.8%), respectively.
Normal normal blood pressure (untreated systolic blood pressure [SBP] < 120 mmHg and diastolic blood pressure [DBP] < 80 mmHg); elevated = elevated blood pressure (untreated SBP 120–129 mmHg and DBP < 80 mmHg); stage 1 = stage 1 hypertension (untreated SBP 130–139 mmHg or DBP 80–89 mmHg); stage 2 = stage 2 hypertension (untreated SBP ≥ 140 mmHg or DBP ≥ 90 mmHg).
During the same follow-up period, 828 central RVO events occurred. The crude cumulative incidence of central RVO in the entire population was 0.071% over 7 years. The cumulative incidence of central RVO over 7 years was lowest in the normal BP group (0.065%), followed by the elevated BP group (0.079%), stage 1 hypertension group (0.12%), and stage 2 hypertension group (0.22%) (Fig. 3). The crude HRs for branch RVO occurrence were 1.82 (1.45–2.29) for elevated BP, 3.45 (2.9–4.1) for stage 1 hypertension, and 6.73 (5.47–8.26) for stage 2 hypertension, compared with normal BP (Table 2). The adjusted HRs for branch RVO occurrence were 1.44 (1.07–1.93) for elevated BP, 2.17 (1.72–2.73) for stage 1 hypertension, and 3.76 (2.91–4.86) for stage 2 hypertension, compared with normal BP (Table 2). The P for trend was <0.001. The population attributable fractions for central RVO for elevated BP, stage 1 hypertension, and stage 2 hypertension were 4.1% (0.3–8.3%), 22.6% (15.3–29.7%), and 14.5% (10.8–18.5%), respectively.
normal = normal blood pressure (untreated systolic blood pressure [SBP] < 120 mmHg and diastolic blood pressure [DBP] < 80 mmHg); elevated = elevated blood pressure (untreated SBP 120–129 mmHg and DBP < 80 mmHg); stage 1 = stage 1 hypertension (untreated SBP 130–139 mmHg or DBP 80–89 mmHg); stage 2 = stage 2 hypertension (untreated SBP ≥ 140 mmHg or DBP ≥ 90 mmHg).
Sensitivity analyses
In the sensitivity analysis, individuals aged <40 and ≥40 years showed similar HRs to the primary analysis (Supplementary Table 1 and Supplementary Table 2). The incidences in individuals aged < 40 years were low and some HRs did not reach statistical significance.
Discussion
The present study using data from a large claims database showed that elevated BP, stage 1 hypertension, and stage 2 hypertension were significantly associated with higher risks for development of RVO and central RVO compared with normal BP. The risks increased stepwise according to the BP groups. Furthermore, the significant positive values of the population attributable fractions for RVO and central RVO indicated that RVO and central RVO could be attributed to stage 1 hypertension and stage 2 hypertension.
Lowering the threshold for hypertension by 10 mmHg was a major revision with great meaning for medicine and public health. Clinical evidence supporting the validity of the 2017 ACC/AHA guideline has accumulated in recent years, especially in the field of cardiology [28, 36,37,38,39]. The present findings showed that individuals with elevated BP and stage 1 hypertension had higher risks of developing RVO than individuals with normal BP, thereby reinforcing the validity of the guideline from the view of ophthalmology.
The cumulative incidences of any RVO were reported to be 3.0% over 9 years in Japan [11], 1.9% over 10 years in China [12], and 1.6% over 10 years in Australia [1]. Compared with these studies, the present study showed lower incidences. This probably arose because the individuals in the present study were younger (mean age after weighting, 48 years) than the individuals in the previous studies (mean age: Japanese study, 60 years; [11] Chinese study, 55 years [12]; Australian study, ≥52 years [1]). Young individuals are generally less likely to develop RVO. In fact, our first sensitivity analysis showed that individuals aged <40 years had fewer RVO events than individuals aged ≥ 40 years. Another explanation for the lower incidences in the present study may be related to differences between the above-reference studies [1, 11, 12] and the present study in terms of data collection. The above studies were population-based complete surveys, which included both individuals with symptomatic RVO and individuals with asymptomatic RVO. In contrast, the present study was based on claims data, which only included data from symptomatic patients with RVO because individuals with asymptomatic RVO do not present to hospitals for treatment; accordingly, they cannot be diagnosed with RVO. Thus, the incidence of RVO may have been lower in the present study than in the previous studies.
Previous studies investigated the association between hypertension severity and RVO occurrence [13, 24]. They found that individuals with uncomplicated hypertension (no end-organ damage) had higher risks for branch RVO and central RVO compared with normotensive individuals, while individuals with complicated hypertension (presence of end-organ damage) had even higher risks. However, the classification of hypertension in these studies was based on ICD-9 codes rather than actual BP measurements. Although a Japanese population-based study used actual BP values to investigate the risk factors for RVO, the number of RVO occurrences was [40] and thus too small to draw any inference [11]. Furthermore, these previous studies did not use the threshold defined by the ACC/AHA guideline. We classified the individuals into normal BP, elevated BP, stage 1 hypertension, and stage 2 hypertension groups using actual BP measurements and the ACC/AHA guideline. Consequently, we obtained the new insight that early-stage hypertension states such as elevated BP and stage 1 hypertension were associated with RVO among young and middle-aged adults.
The present results suggest that elevated BP should be considered with caution from an early stage, which was not regarded as a treatment target before the publication of the 2017 ACC/AHA guideline [14]. Doctors should encourage patients to make healthy choices with regard to their diet and physical activity as recommended by the guideline. Efforts to change lifestyle factors can reduce the risk for cardiovascular diseases as previously described [39]. Such efforts would also decrease the risk for RVO, leading to preservation of visual acuity and improved quality of life. Fewer RVO events will reduce the physical, psychological, and financial burden on patients. Moreover, healthcare costs would be saved because of the reduced need to use expensive intravitreal anti-vascular endothelial growth factor drugs for treatment of macular oedema caused by RVO.
The population attributable fractions for RVO were highest in the stage 1 hypertension group. These results indicate that the stage 1 hypertension group would have the most influence on the population if BP was made normal in all individuals. For example, 20.6% of RVO occurrences would be prevented if these individuals did not have stage 1 hypertension (i.e. had normal BP). Thus, we need to pay attention to not only stage 2 hypertension but also stage 1 hypertension to prevent RVO occurrence. However, population attributable fractions assume that there is a perfect intervention to eradicate the exposure [15]. This assumption is often unrealistic. Thus, in future studies, we need to investigate the extent of the effect that decreasing BP has on individuals with early-stage hypertension using real-world data.
One of the strengths of the present study is that we were able to obtain specific numerical data on BP. Furthermore, we were able to follow individuals in a very large sample size longitudinally over a long period of time. Given the relatively low incidence of RVO, performance of randomised controlled studies would be difficult. Thus, the findings obtained in this observational study are useful for filling the evidence gap.
There are several limitations to the present study. First, because almost all of the individuals in the database were working-age Asians, the results may not be generalizable to other races, ethnicities, and education levels. However, given that a meta-analysis did not show associations between races and prevalence of RVO [2], racial differences may not be very important. Second, BP was measured in individuals on a single occasion during a health checkup. Therefore, this BP may not fully reflect the BP phenotype of the individuals. For example, BP measured during health checkups may have been higher than that measured at home. A case-control study reported an association between RVO and BP variability [41], and thus further studies with measurement of 24-hour ambulatory BP are warranted. Third, the protocol for BP measurement, established by the Japanese Ministry of Health, Labour and Welfare, may not have been completely followed in real-world settings. Fourth, the prevalence of RVO was lower in the present study than in previous population-based complete surveys [1, 11, 12]. This difference is presumably because the present study, which used claims data, could not include individuals with asymptomatic RVO, while the complete surveys could include such individuals. However, comparisons of different BP groups in a limited population with symptomatic RVO would be valid. Fifth, the codes of claims data are not entirely accurate. A validation study in Japan showed that the diagnosis of age-related macular degeneration in claims data had a high sensitivity (94.9%) and a high specificity (92.6%) [40]; however, such validation studies have not been performed with regard to RVO. In some RVO cases, the RVO diagnostic codes may not have been registered because the patients did not require treatment; this phenomenon may have contributed to underestimation of the incidence. However, we believe that the specificity of the RVO codes was relatively high; comparisons of different BP groups in a limited population with RVO codes would be valid.
In conclusion, occurrence of RVO and central RVO was found to be associated with elevated BP and stage 1 hypertension. The BP categorization advocated by the 2017 ACC/AHA guideline can be useful for identifying young and middle-aged adults at increased risk for RVO.
Summary
What was known before
-
One of the major and modifiable risk factors for retinal vein occlusion is hypertension.
-
The threshold of hypertension was lowered to 130/80 mmHg by the 2017 American College of Cardiology/American Heart Association blood pressure guideline, but the association between early-stage hypertension and retinal vein occlusion is unknown.
What this study adds
-
Using a large administrative claims database, we found that even early-stage hypertension was associated with the occurrence of retinal vein occlusion.
Data availability
We used de-identified, individual-level data obtained from the JMDC Claims Database (Tokyo, Japan). The address of their HP is https://www.jmdc.co.jp/en/index. Data are not publicly available.
References
Cugati S, Wang JJ, Rochtchina E, Mitchell P. Ten-year incidence of retinal vein occlusion in an older population: The Blue Mountains Eye Study. Arch Ophthalmol. 2006;124:726–32.
Rogers S, McIntosh RL, Cheung N, Lim L, Wang JJ, Mitchell P, et al. The Prevalence of Retinal Vein Occlusion: Pooled Data from Population Studies from the United States, Europe, Asia, and Australia. Ophthalmology 2010;117:313–319.e1.
United Nations Population Fund. World Population Dashboard. Available at: https://www.unfpa.org/data/world-population-dashboard.
Korobelnik J-F, Larsen M, Eter N, Bailey C, Wolf S, Schmelter T, et al. Efficacy and safety of intravitreal aflibercept treat-and-extend for macular edema in central retinal vein occlusion: the centera study. Am J Ophthalmol. 2021;227:106–15.
Clark WL, Boyer DS, Heier JS, Brown DM, Haller JA, Vitti R, et al. Intravitreal aflibercept for macular edema following branch retinal vein occlusion 52-week results of the vibrant study. Ophthalmology 2016;123:330–6.
Heier JS, Campochiaro PA, Yau L, Li Z, Saroj N, Rubio RG, et al. Ranibizumab for macular edema due to retinal vein occlusions: Long-term follow-up in the HORIZON trial. Ophthalmology 2012;119:802–9.
Brown DM, Campochiaro PA, Singh RP, Li Z, Gray S, Saroj N, et al. Ranibizumab for Macular Edema following Central Retinal Vein Occlusion. Six-Month Primary End Point Results of a Phase III Study. Ophthalmology 2010;117:1124–1133.e1.
Hykin P, Prevost AT, Vasconcelos JC, Murphy C, Kelly J, Ramu J, et al. Clinical Effectiveness of Intravitreal Therapy With Ranibizumab vs Aflibercept vs Bevacizumab for Macular Edema Secondary to Central Retinal Vein Occlusion: A Randomized Clinical Trial. JAMA Ophthalmol. 2019;137:1256–64.
Kymes SM, Vollman D. Recognizing the true cost of medical spending—an assessment of ranibizumab for retinal disorders. JAMA Ophthalmol. 2019;137:1432–3.
Kolar P. Risk factors for central and branch retinal vein occlusion: a meta-analysis of published clinical data. J Ophthalmol. 2014;2014:1–5.
Arakawa S, Yasuda M, Nagata M, Ninomiya T, Hirakawa Y, Doi Y, et al. Nine-year incidence and risk factors for retinal vein occlusion in a general japanese population: the hisayama study. Investig Opthalmology Vis Sci. 2011;52:5905–9.
Zhou JQ, Xu L, Wang S, Wang YX, You QS, Tu Y, et al. The 10-year incidence and risk factors of retinal vein occlusion. Ophthalmology 2013;120:803–8.
Stem MS, Talwar N, Comer GM, Stein JD. A longitudinal analysis of risk factors associated with central retinal vein occlusion. Ophthalmology 2013;120:362–70.
Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Dennison Himmelfarb C, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018;71:1269–324.
Mansournia MA, Altman DG. Population attributable fraction. BMJ. 2018;360:k757. https://doi.org/10.1136/bmj.k757.
Nagai K, Tanaka T, Kodaira N, Kimura S, Takahashi Y, Nakayama T. Data resource profile: JMDC claims database sourced from health insurance societies. J Gen Fam Med. 2021;22:118–27.
JMDC Inc. Available at: https://www.jmdc.co.jp/en/jmdc-claims-database/ [Accessed July 26, 2021].
Anon. Risk factors for central retinal vein occlusion. The Eye Disease Case-Control Study Group. Arch Ophthalmol 1996;114:545–54.
Mitchell P, Smith W, Chang A. Prevalence and Associations of Retinal Vein Occlusion in Australia: The Blue Mountains Eye Study. Arch Ophthalmol. 1996;114:1243–7.
Lee KS, Nam KH, Kim DW, Kang EC, Koh HJ. Risk of Retinal Vein Occlusion in Patients With End-Stage Renal Disease: A 12-Year, Retrospective, Nationwide Cohort Study in South Korea. Investig Opthalmology Vis Sci. 2018;59:39–44.
Rehak M, Rehak J, Müller M, Faude S, Faude F, Siegemund A, et al. The prevalence of activated protein C (APC) resistance and factor V Leiden is significantly higher in patients with retinal vein occlusion without general risk factors: Case-control study and meta-analysis. Thromb Haemost. 2008;99:925–9.
Ministry of Health, Labour and Welfare. Standard Medical Examination / Health Guidance Program. Available at: https://www.mhlw.go.jp/bunya/shakaihosho/iryouseido01/info03a.html [Accessed August 7, 2021].
Paik DW, Han K, Kang SW, Ham D-I, Kim SJ, Chung T-Y, et al. Differential effect of obesity on the incidence of retinal vein occlusion with and without diabetes: a Korean nationwide cohort study. Sci Rep. 2020;10:10512.
Newman-Casey PA, Stem M, Talwar N, Musch DC, Besirli CG, Stein JD. Risk factors associated with developing branch retinal vein occlusion among enrollees in a united states managed care plan. Ophthalmology 2014;121:1939–48.
Klein R, Klein BE, Moss SE, Meuer SM. The epidemiology of retinal vein occlusion: the Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2000;98:133–43.
Sperduto RD, Hiller R, Chew E, Seigel D, Blair N, Burton TC, et al. Risk factors for hemiretinal vein occlusion: comparison with risk factors for central and branch retinal vein occlusion: the eye disease case-control study. Ophthalmology 1998;105:765–71.
Shahsuvaryan ML, Melkonyan AK. Central retinal vein occlusion risk profile: a case-control study. Eur J Ophthalmol. 2003;13:445–52.
Kaneko H, Yano Y, Itoh H, Morita K, Kiriyama H, Kamon T, et al. Association of Blood Pressure Classification Using the 2017 American College of Cardiology/American Heart Association Blood Pressure Guideline With Risk of Heart Failure and Atrial Fibrillation. Circulation 2021;143:2244–53.
Austin PC. Using the Standardized Difference to Compare the Prevalence of a Binary Variable Between Two Groups in Observational Research. Commun Stat - Simul Comput. 2009;38:1228–34.
Pocock SJ, Clayton TC, Altman DG. Survival plots of time-to-event outcomes in clinical trials: good practice and pitfalls. Lancet. 2002;359:1686–9.
Patino CM, Ferreira JC. Test for trend: evaluating dose-response effects in association studies. J Bras Pneumol. 2016;42:240–240.
Hess KR. Graphical methods for assessing violations of the proportional hazards assumption in cox regression. Stat Med. 1995;14:1707–23.
Therneau T, Crowson C, Atkinson E Using time dependent covariates and time dependent coefficients in the cox model. 2022. Available at: https://cran.r-project.org/web/packages/survival/vignettes/timedep.pdf.
Yoshida K, Hernández-Díaz S, Solomon DH, Jackson JW, Gagne JJ, Glynn RJ, et al. Matching weights to simultaneously compare three treatment groups: Comparison to three-way matching. Epidemiol Camb Mass. 2017;28:387–95.
Natarajan S, Lipsitz SR, Rimm E. A simple method of determining confidence intervals for population attributable risk from complex surveys. Stat Med. 2007;26:3229–39.
Yano Y, Reis JP, Colangelo LA, Shimbo D, Viera AJ, Allen NB, et al. Association of Blood Pressure Classification in Young Adults Using the 2017 American College of Cardiology/American Heart Association Blood Pressure Guideline With Cardiovascular Events Later in Life. JAMA 2018;320:1774–82.
Son JS, Choi S, Kim K, Kim SM, Choi D, Lee G, et al. Association of Blood Pressure Classification in Korean Young Adults According to the 2017 American College of Cardiology/American Heart Association Guidelines With Subsequent Cardiovascular Disease Events. JAMA. 2018;320:1783–92.
Matsuoka S, Kaneko H, Yano Y, Itoh H, Fukui A, Morita K, et al. Association Between Blood Pressure Classification Using the 2017 ACC/AHA Blood Pressure Guideline and Retinal Atherosclerosis. Am J Hypertens. 2021;34:1049–56.
Jones DW, Whelton PK, Allen N, Clark D, Gidding SS, Muntner P, et al. Management of Stage 1 Hypertension in Adults With a Low 10-Year Risk for Cardiovascular Disease: Filling a Guidance Gap: A Scientific Statement From the American Heart Association. Hypertension 2021;77:e58–e67.
Tamiya R, Miyake M, Kido A, Hiragi S, Tamura H, Kuroda T, et al. Validation study of the claims-based definition for age-related macular degeneration at a single university hospital in Japan. Jpn J Ophthalmol. 2021;65:388–94.
Gulmez M, Tekce A. Blood pressure variability in patients with branch retinal vein occlusion. Retina 2020;40:2045–9.
Funding
This work was supported by grants from the Ministry of Health, Labour and Welfare, Japan (21AA2007) and the Ministry of Education, Culture, Sports, Science and Technology, Japan (20H03907 and 21H03159). The sponsor or funding organization had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Contributions
YH had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: YH, HK, AO, HY, and RO. Acquisition, analysis, and interpretation of data: YH, HK, SA, AO, HM, HY, RO. Drafting of the manuscript: YH, HK, HY, and AO. Critical revision of the manuscript for important intellectual content: YH. Statistical analysis: YH, SA, AO, and HY. Obtained funding: HY. Administrative, technical, or material support: None. Study supervision: HY and AO.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
41433_2022_2241_MOESM3_ESM.docx
Hazard ratios for any retinal vein occlusion and central retinal vein occlusion before and after weighting in the individuals aged <40 years
41433_2022_2241_MOESM4_ESM.docx
Hazard ratios for any retinal vein occlusion and central retinal vein occlusion before and after weighting in the individuals aged ≥40 years
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hashimoto, Y., Kaneko, H., Aso, S. et al. Association between retinal vein occlusion and early-stage hypertension: A propensity score analysis using a large claims database. Eye 37, 1741–1747 (2023). https://doi.org/10.1038/s41433-022-02241-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02241-w
- Springer Nature Limited