Abstract
Background/Objectives
To report the impact of interventions for avoidable vision impairment (VI) on the visual function of elderly residents in ‘homes for the aged’ in India.
Methods
Participants aged ≥60 years were recruited. A comprehensive eye examination was conducted by trained examiners and interventions were provided. Trained social investigators administered the Indian Vision Function questionnaire (INDVFQ) to assess visual function before and after the intervention (spectacles, cataract surgery or laser capsulotomy). Lower scores on IVFQ imply better visual function. VI was defined as presenting visual acuity worse than 6/18 in the better eye. VI due to cataract, uncorrected refractive errors, and posterior capsular opacification after cataract surgery were considered avoidable VI.
Results
The mean age of the participants (n = 613) was 73.8 years (standard deviation: 8.1 years) and 378 (62.2%) were women. 64/103 (62.1%) participants who had avoidable VI at baseline were evaluated after the intervention. Significant gains were observed in all four domains of visual function. There was a 14.9% improvement in mobility scores (33.8 versus 28.8; p = 0.03), a 19.9% improvement in the activity limitations score (36.8 versus 29.5; p < 0.01), a 10.9% improvement in the psychosocial impact score (41.1 versus 36.6; p < 0.01) and a 13.6% improvement in the visual symptoms score (49.2 versus 42.5 p < 0.01). Overall, the mean IVFQ score improved by 16.4% (47.6 versus 39.8; p < 0.01).
Conclusion
Elderly individuals in residential care with avoidable VI had a significant improvement in visual function after relatively low-cost interventions such as spectacles and cataract surgery. Strategies are needed to provide these interventions for the elderly in ‘homes for the aged’ in India.
Similar content being viewed by others
Introduction
Vision loss is a public health challenge affecting over a billion people worldwide [1]. Over 75% of visual impairment (VI) is avoidable [2]. VI is more common among the elderly living in residential care when compared to those living in their own homes in non-institutionalized environments [3,4,5,6]. A significant portion of VI among the elderly can be corrected by low cost interventions such as spectacles and cataract surgery [7,8,9,10]. Studies have shown that VI in the elderly affects all dimensions of their life, including mobility, self-care, driving, participation in social and religious activities and overall quality of life [11,12,13,14].
Patient-reported outcome measures are increasingly emphasized in addition to visual acuity to assess the impact of interventions [15]. There are few studies on VI among the elderly in residential care in India [16], however longitudinal studies on visual function and the impact of interventions on visual function have not been reported. Research in this area is needed as there is an increase in the number of elderly people living in ‘homes for the aged’ due to demographic and societal changes in India [17, 18].
The Hyderabad Ocular Morbidity in Elderly Study (HOMES) is a longitudinal study with pre and post-intervention phases designed to: (a) investigate the prevalence, causes, and risk factors for VI among elderly individuals living in residential care facilities in Hyderabad, India, and (b) assess the impact of interventions such as spectacles and cataract surgery on visual function in elderly living in residential care. We have reported a 30.1% prevalence of VI in this study population at the baseline examination [19]. In this paper, we report on the impact of interventions for avoidable vision loss on visual function in the elderly living in residential care.
Materials and methods
Ethics approval
The institutional review board of the Hyderabad Eye Research Foundation, L V Prasad Eye Institute, Hyderabad, India approved the study protocol, and the study protocols conformed to the tenets outlined in the Declaration of Helsinki. All participants provided written informed consent [20]. HOMES was a longitudinal study that was carried out in the ‘homes of the aged’ residential care facilities with pre and post-intervention phases, carried out in Hyderabad (a city in the south Indian state of Telangana) and its surrounding regions during 2017 and 2019 [20]. In total, 1513 participants aged 60 years and older were enumerated from 41 residential homes, of which, 1182 (78.1%) were examined [16]. Of these, 867 had Hindi Mini-Mental Examination (HMSE) scores of more than 20 and were considered for comprehensive assessment including assessment of visual function. [20, 21].
Questionnaire
At baseline and follow-up, trained investigators administered questionnaires in the local language (Telugu). These questionnaires included personal, sociodemographic, ocular, and systemic history and the previously validated Indian Vision Function Questionnaire (INDVFQ) [22, 23]. Participants who were bedridden or had issues related to mobility were examined and included as part of the study, but not all the questionnaires, including INDFVQ were administered to them. After collecting personal and demographic information, the Hindi Mini-mental State Examination (HMSE) assessment questionnaire was administered. If the HMSE score was less than 20 (suggestive of a mild cognitive impairment), the evaluation was restricted to systemic and ocular history, disease risk factors, and clinical examinations. For all individuals whose HMSE scores were greater than or equal to 20, the INDVFQ was administered, and a complete examination was conducted [20].
INDVFQ was used to assess self-reported visual function. This 33-item questionnaire was validated in the Indian population and it measures four dimensions of visual function, mobility, activity limitation, psychosocial impact, and visual symptoms [22,23,24,25]. We validated the INDVFQ and also reported the impact of vision loss in the elderly population in residential care [14, 26]. In the INDVFQ, questions 1–22 are scored on a 5-point Likert scale, and the remaining 11 questions are scored on a 4-point scale. Options 1–4 on both scales are identical, and only option 5 for questions 1–22 differs with the addition of “cannot do this because of my sight”. A higher score on the scale represents a higher degree of difficulty. We excluded six questions that had more than 10% missing responses and/or not applicable to the elderly in the residential care: Question 1 (difficulty climbing stairs), Question 4 (difficulty finding the way in new places), Questions 5 (difficulty going to social functions like weddings), Question 8 (difficulty seeing the step of a bus climbing in or out), Question 13 (difficulty doing work up to usual standard) and Question 23 (difficulty to enjoy social functions). Individuals who were bedridden or confined to a wheelchair, as well as those who needed assistance to walk were excluded from this study as few INDVFQ questions focus on mobility.
Clinical examination protocol
The clinical examination protocol is described in our previous publication [20]. In brief, visual acuity (VA) was measured for distance with standard logMAR (logarithm of the minimum angle of resolution) charts with tumbling E or letter optotypes under good illumination of at least 180 lux measured using a light meter. Near vision was assessed using a logMAR chart with tumbling E or letter optotypes at a fixed distance of 40 cm. The visual acuity for both distance and near was tested with the participant’s presenting refractive correction where spectacles were used. Anterior segment examination was carried out using a portable slit lamp (BA 904 Haag-Streit Clement Clarke International, UK). Fundus examination and retinal photography were conducted using a non-mydriatic fundus camera (Visuscout 100 Handheld Fundus Camera, Carl Zeiss Meditec, USA) on all participants.
Definitions
Distance vision impairment (DVI) was defined as presenting visual acuity worse than 6/18 in the better eye of a participant. VI caused by cataract, uncorrected refractive error, or posterior capsular opacification was considered avoidable VI. DVI was further classified as Moderate VI (<6/18 to 6/60 in the better-seeing eye) and Severe VI/Blindness (<6/60 to no perception of light in the better-seeing eye). Near vision impairment (NVI) was defined as presenting binocular near vision worse than N8 and no DVI.
Intervention for visual impairment
All participants with VI in one or both eyes were referred to L V Prasad Eye Institute for a comprehensive eye examination. All services, including eye examinations, surgeries, ophthalmic lasers, and spectacles, were provided at no-cost to the participants. Clinical consultations at the institute were facilitated by study staff and based on the need, assistance for transportation was provided. The clinical protocol and questionnaires were administered twice to all participants, once at baseline and then again 6 to 9 months after care was provided.
Data management
Data for each participant were recorded on HOMES data collection forms and entered in the database developed in Microsoft Access with built-in validation checks. Data analysis was conducted using Stata Statistical Software for Windows, version 14. The sub-scales score for each of the INDVFQ domains was calculated as the sum of the response scores divided by the maximum possible score and multiplied by 100 to get a domain score. Similarly, the overall INDVFQ score was calculated as the simple mean of the responses for each of the questions. A similar methodology has been used previously [24, 27, 28]. Paired t-tests were used to compare the mean scores for each domain and overall INDVFQ scores pre and post-intervention. The Chi-square test was used to compare the statistical difference for categorical variables. Pearson correlation coefficients were calculated to estimate the linear relationship between VA (in logMAR) change and INDVFQ scores pre and post-intervention.
Results
Characteristics of the participants
In total, 867 participants were examined at baseline and 613 (70.7%) participants were available at follow-up visit. Of the 225 participants who were examined at baseline but not examined at follow-up, 133 (59.1%) were no longer living in the residential homes and could not be reached, 54 (24.0%) refused participation, and 38 (16.9%) participants expired before the follow-up examination. Those examined and not examined at follow-up were similar in terms of mean age (p = 0.20) and gender (p = 0.77). In addition, 15 participants who developed mobility issues and 14 participants with a decline in HMSE scores compared to baseline were excluded from the analysis. The data of the remaining 613/867 (70.7%) participants was included in the final analysis. The median time between baseline and the post-intervention visit was eight months (mean ± SD: 8.1 ± 4 months). The follow-up questionnaire was administered at least six weeks after intervention.
The mean age of the participants (n = 613) was 73.8 years (standard deviation: 8.1 years) and 378 (62.2%) participants were women. The mean duration of stay in the homes was 4.7 years (SD:4.9 years). In total, 482/613 (78.6%) participants were using spectacles at the baseline visit. Most of them were using bifocals (88%). In terms of co-morbidities, 185 (30.2%) participants had diabetes and 367 (59.9%) participants had hypertension.
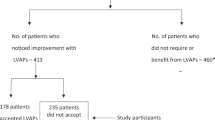
In total, 212 participants had either avoidable DVI (n = 103) or NVI (n = 109) at baseline visit. (Fig. 1). In all, 125/212 (59%) participants were provided with an intervention, including spectacles in 80 (64%) participants and 45 (36%) participants who received cataract surgery (n = 41) or YAG laser capsulotomy (n = 4). Older participants and those living in paid homes for the aged centres had a significantly lower uptake of the intervention. (Table 1) The uptake of the intervention was not associated with gender or level of education. (p = 0.35, Table 1)
Impact of interventions
At the post-intervention visit, 64/103 (62.1%) participants who had DVI at baseline were available for examination. Significant gains were observed in all four domains of visual function post-intervention with lower scores post-intervention suggestive of better visual function. There was 14.9% improvement in mobility scores (33.8 versus 28.8; p = 0.03), 19.9% improvement in the activity limitations score (36.8 versus 29.5; p < 0.01), a 10.9% improvement in the psychosocial impact score (41.1versus 36.6; p < 0.01) and a 13.6% improvement in visual symptoms score (49.2 versus 42.5 p < 0.01, Table 2). Overall, the mean IVFQ score improved by 16.4% (47.6 versus 39.8; p < 0.01).
Among those who had NVI at baseline (n = 61), significant gains were observed in two of the four domains, with the greatest improvement of 11.2% in visual symptoms score (42.7 versus 37.9; p = 0.02) followed by 7.5 % in activity limitation (28.0 versus 25.9; p = 0.02). There was no statistically significant change in scores for mobility and psychosocial impact score between the baseline and post-intervention.
In terms of the type of intervention provided and visual function scores, among those provided with spectacles (n = 80) significant improvement in INDVFQ scores, were noted only for activity limitation (29.4 versus 26.8; p < 0.01) along with an overall 6.9% improvement in the INDVFQ score (39.1 versus 36.4; p = 0.02, Table 3).
Improvement in visual function scores was higher among those who had surgical interventions (n = 45) compared to spectacles intervention. This included 4 participants who had YAG capsulotomy for posterior capsular opacification as well as those who had cataract surgery. Activity limitation scores improved by 22.6% (38.1 versus 29.5; p < 0.01), followed by 21% improvement in visual symptoms (52.6 versus 41.5), 19.8% improvement in mobility scores (36.4 versus 29.2; p < 0.01) and 16.2% improvement in psychosocial domains (40.7 versus 34.1; p < 0.01). Overall, an 21% change was observed in INDVFQ scores (50.0 versus 39.5; p < 0.01, Table 3).
In terms of severity of VI and improvement in visual function, among the participants who had moderate VI at baseline (n = 58), the mean IVFQ score improved by 14.4% after the intervention (45.9 versus 39.3; p < 0.001) with even greater improvement among those who had Severe VI/Blindness (mean INDVFQ score improved by 31% after the intervention, 63.5 versus 43.8; p < 0.001). Based on presenting visual acuity definition including mild visual impairment, defined as presenting vision worse than 6/12 in the better eye, data on INDVFQ score was available from 138/255 participants. Overall, 10.3% improvement was observed in INDVFQ scores post intervention compared to the baseline score (42.7 versus 38.3; p < 0.01).
The impact of intervention on INDVFQ score showed gender differences before and after intervention. Overall, the percentage score was higher among women compared to men (8.9% versus 15.9%; p < 0.01). (supplementary material)
The mean baseline visual acuity in the better eye among those who had DVI was 0.67 log MAR (95% CI: 0.61–0.73) which improved to 0.41 log MAR (0.36–0.48) after the intervention. Among those who were dispensed spectacles, the mean baseline visual acuity in the better eye was 0.62 logMAR (95% CI:0.54–0.70 which improved to 0.46 (95% CI: 0.36–0.56), equivalent 1.5 lines on logMAR visual acuity post-intervention. Among those who had cataract surgery intervention, the mean baseline visual acuity was 0.70 logMAR (95% CI: 0.62–0.78) which improved to 0.38 log MAR (95% CI: 0.31–0.46), equivalent to over three lines of improvement on the logMAR visual acuity chart post-intervention (Table 4).
Discussion
Interventions to address DVI and NVI in residents of homes for the aged in India had a significant positive impact on self-reported visual function. There was substantial improvement in the domains of activity limitation and visual symptoms and less improvement for mobility. We have earlier reported that reading, watching television, and helping in household work are viewed as the most important tasks in which the elderly in these homes engage, indicating that the reported improvements were highly valued [29]. Both spectacles and cataract surgery resulted in improved visual function scores in the elderly, but cataract surgery had an even larger impact. Most individuals in the current study were confined to the homes in which they lived, and therefore had limited mobility beyond these facilities.
The psychosocial domain of the INDVFQ appeared to improve the least in response to the provision of care for vision loss. The five questions in the INDVFQ in the psychosocial domain may be inadequate to assess the impact of the intervention on social and emotional wellbeing. Other study in India have also reported a similar finding [23]. There could be other factors associated with emotional wellbeing (e.g., isolation, lack of family care) among the elderly in residential care that drive the responses to these questions, and a more elaborate assessment may be required to identify any improvements gained from better vision.
The impact of cataract surgery was significantly higher in comparison to the provision of spectacles for vision loss for all domains. Earlier studies have reported that self-reported visual function scores are worse among people with cataract compared to those with uncorrected refractive errors [28, 30, 31]. It is likely that the quality of vision is worse in cataract compared to that of uncorrected refractive errors [14, 31]. We also noted that there was an over three-line improvement in visual acuity in the cataract surgery intervention group compared to only one line and half line improvement in the spectacles group. This difference in net visual acuity gain post-intervention could partly explain the difference in visual function score between the two interventions. In our earlier report from the same study, we noted that visual function was worse among those who had cataract as a cause of VI when compared to those with uncorrected refractive errors [14]. Others have also shown improvement in visual function after cataract surgery similar to the findings of our study [24, 32, 33]. One advantage of the present study is that previous studies reporting on the adverse impact of vision loss on visual function were mostly cross-sectional in design or included patients presenting to hospitals, whereas this study was prospective and wholly focused on residents of homes for the aged. [28, 30, 23, 33, 34]. Though the impact of cataract surgery is evident across the studies, the magnitude of impact may not be directly comparable to our study due to differences in participants.
We used the INDVFQ questionnaire, an instrument was validated in the Indian population for assessing visual function. However, the INDVFQ was developed for assessing the impact of VI due to cataract [23, 33]. It is possible that the smaller impact of the correction of refractive errors is not detected with this questionnaire owing to the nature of the questionnaire and also magnitude of vision loss due to refractive errors. Also, Finger and colleagues found that that INDVFQ is more sensitive in severe vision loss [23]. Furthermore, we had to exclude six questions as they were not applicable to the study population.
HOMES is one of very few longitudinal studies undertaken to assess vision loss among elderly individuals living in residential care. Assessment of visual acuity in standard testing conditions, interviews by well-trained personnel in the comfort of the homes, voice recording of interviews and review of randomly selected interviews, are all strengths of our study. One important limitation of our study was the poor uptake of services among those referred for care. The uptake of services was lower in private homes largely because we were unable to get permission from home authorities or the kin of the participants to use referral services provided directly by the LV Prasad Eye Institute. The barriers for uptake of eye care services among the elderly in residential care were reported in the recent publication [35].
Also, among those referred, cataract surgery could not always be performed as participants, or their kin elected to defer surgery. All these factors resulted in a smaller sample of participants post-intervention and may also bias the results towards greater impact as those accepting services may have noticed greater limitations than those who chose to defer. Also, there were more participants in the 60-69 years age group which is attributed to the inclusion criteria used in the report and also a lower uptake of interventions in the older age groups. Finally, the study findings are not generalizable to elderly individuals living independently in the community as all the participants included in this study lived in residential care.
In summary, relatively simple and cost-effective interventions, such as cataract surgery, spectacles, and YAG capsulotomy, resulted in significant improvement in self-reported visual function among elderly residents of homes for the aged centres in India. Strategies are needed to reach out to this population to provide interventions and care as vision loss is common and a large proportion of vision loss is avoidable. Challenges for the uptake of services also need to be addressed [35]. Not only the provision of cataract surgery but also periodic follow-up-care is essential to provide good vision. We previously reported the large burden of vision loss after cataract surgery due to uncorrected refractive errors and posterior capsular opacification in this population [36].
We propose an elderly centric eye care model on similar lines to the school eye model programme where annual eye health assessments can be conducted in the homes and followed with provision of services [19]. This strategy can be adopted by the national programme for control of blindness in India and other non-governmental organisations working elderly health care. A cross-subsidy model where elderly in paying homes can pay for services and also support services for those in free homes may be experimented for long term sustainability. Evaluation in residential facilities may help overcome barriers to travel and improve the uptake of services. These comprehensive assessments can be accomplished largely using teleophthalmology as is now done in vision centres and elsewhere [37]. Appropriate referrals can be made for surgical interventions as needed, but spectacles can be provided on-site. Such a model is being introduced in parts of India [37]. Wider replication of this model will contribute to the overarching goal of healthy aging in India.
Summary
What was known before
-
There is no data on the impact of interventions on the visual function of the elderly in residential care in India.
What this study adds
-
Cataract surgery and spectacles improves visual function of the elderly in residential care in India
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Bourne R, Steinmetz JD, Flaxman S, Briant PS, Taylor HR, Resnikoff S, et al. Trends in prevalence of blindness and distance and near vision impairment over 30 years: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9:e130–43.
Steinmetz JD, Bourne RRA, Briant PS, Flaxman SR, Taylor HRB, Jonas JB, et al. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9:e144–60.
Mitchell P, Hayes P, Wang JJ. Visual impairment in nursing home residents: the Blue Mountains Eye Study. Med J Aust. 1997;166:73–76.
Owsley C, McGwin G, Scilley K, Meek GC, Dyer A, Seker D. The visual status of older persons residing in nursing homes. Arch Ophthalmol. 2007;125:925–30.
Sinoo MM, Kort HS, Duijnstee MS. Visual functioning in nursing home residents: information in client records. J Clin Nurs. 2012;21:1913–21.
Eichenbaum JW, Burton WB, Eichenbaum GM, Mulvihill M. The prevalence of eye disease in nursing home and non-nursing home geriatric populations. Arch Gerontol Geriatrics. 1999;28:191–204.
Evans BJ, Rowlands G. Correctable visual impairment in older people: a major unmet need. Ophthalmic Physiol Opt. 2004;24:161–80.
Foran S, Rose K, Wang JJ, Mitchell P. Correctable visual impairment in an older population: the blue mountains eye study. Am J Ophthalmol. 2002;134:712–9.
Fung MM, Yap M, Cheng KK. Correctable visual impairment among people with diabetes in Hong Kong. Clin Exp Optom. 2010;93:453–7.
Hollands H, Brox AC, Chang A, Adilman S, Chakraborti B, Kliever G, et al. Correctable visual impairment and its impact on quality of life in a marginalized Canadian neighbourhood. Can J Ophthalmol. 2009;44:42–48.
Lamoureux EL, Fenwick E, Moore K, Klaic M, Borschmann K, Hill K. Impact of the severity of distance and near-vision impairment on depression and vision-specific quality of life in older people living in residential care. Invest Ophthalmol Vis Sci. 2009;50:4103–9.
Varma R, Wu J, Chong K, Azen SP, Hays RD, Los Angeles Latino Eye Study G. Impact of severity and bilaterality of visual impairment on health-related quality of life. Ophthalmology. 2006;113:1846–53.
Jacobs JM, Hammerman-Rozenberg R, Maaravi Y, Cohen A, Stessman J. The impact of visual impairment on health, function and mortality. Aging Clin Exp Res. 2005;17:281–6.
Marmamula S, Mitchell W, Zebardast N, Locascio J, Barrenkala NR, Kumbham TR, et al. Impact of vision loss on visual function among elderly residents in the “Home for the Aged” in India: the hyderabad ocular morbidity in elderly study. Transl Vis Sci Technol. 2020;9:11.
Snyder CF, Jensen RE, Segal JB, Wu AW. Patient-reported outcomes (PROs): putting the patient perspective in patient-centered outcomes research. Med Care. 2013;51:S73–79.
Marmamula S, Ravuri CS, Boon MY, Khanna RC. A cross-sectional study of visual impairment in elderly population in residential care in the South Indian state of Andhra Pradesh: a cross-sectional study. BMJ Open. 2013;3:e002576.
Mane AB. Ageing in India: some social challenges to elderly care. J Gerontol Geriatr Res. 2016;5:e136.
Yeolekar ME. Elderly in India-needs and issues. J Assoc Physicians India. 2005;53:843–4.
Marmamula S, Barrenakala NR, Challa R, Kumbham TR, Modepalli SB, Yellapragada R, et al. Prevalence and risk factors for visual impairment among elderly residents in ‘homes for the aged’ in India: the Hyderabad Ocular Morbidity in Elderly Study (HOMES). Br J Ophthalmol. 2021;105:32–36.
Marmamula S, Barrenkala NR, Challa R, Reddy K T, Yellapragada S, Brahmanandam M S, et al. Hyderabad ocular morbidity in elderly study (HOMES) - rationale, study design and methodology. Ophthalmic Epidemiol. 2020;27:83–92.
Ganguli M, Ratcliff G, Chandra V, Sharma S, Gilby J, Pandav R, et al. A Hindi version of the MMSE: the development of a cognitive screening instrument for a largely illiterate rural elderly population in India. Int J Geriatr psychiatry. 1995;10:367–77.
Gothwal VK, Bagga DK, Sumalini R. Rasch analysis of the Indian vision function questionnaire. Br J Ophthalmol. 2012;96:619–23.
Finger RP, Kupitz DG, Holz FG, Balasubramaniam B, Ramani RV, Lamoureux EL, et al. The impact of the severity of vision loss on vision-related quality of life in India: an evaluation of the IND-VFQ-33. Invest Ophthalmol Vis Sci. 2011;52:6081–8.
Gupta SK, Viswanath K, Thulasiraj RD, Murthy GV, Lamping DL, Smith SC, et al. The development of the Indian vision function questionnaire: field testing and psychometric evaluation. Br J Ophthalmol. 2005;89:621–7.
Murthy GV, Gupta SK, Thulasiraj RD, Viswanath K, Donoghue EM, Fletcher AE. The development of the Indian vision function questionnaire: questionnaire content. Br J Ophthalmol. 2005;89:498–503.
Mitchell W, Marmamula S, Zebardast N, Ng W, Locascio JJ, Kumbam T, et al. Psychometric validation techniques applied to the IND-VFQ-33 visual function questionnaire: the Hyderabad ocular morbidity in the elderly study (HOMES). BMC Med Res Methodol. 2021;21:26.
Nutheti R, Shamanna BR, Nirmalan PK, Keeffe JE, Krishnaiah S, Rao GN, et al. Impact of impaired vision and eye disease on quality of life in Andhra Pradesh. Invest Ophthalmol Vis Sci. 2006;47:4742–8.
Nirmalan PK, Tielsch JM, Katz J, Thulasiraj RD, Krishnadas R, Ramakrishnan R, et al. Relationship between vision impairment and eye disease to vision-specific quality of life and function in rural India: the Aravind Comprehensive Eye Survey. Invest Ophthalmol Vis Sci. 2005;46:2308–12.
Marmamula S, Barrenkala NR, Khanna RC, Challa R, Bhakki M, Kumbham TR, et al. Near vision impairment among the elderly in residential care-the Hyderabad Ocular Morbidity in Elderly Study (HOMES). Eye (Lond). 2021;35:2310–15.
Nutheti R, Keeffe JE, Shamanna BR, Nirmalan PK, Krishnaiah S, Thomas R. Relationship between visual impairment and eye diseases and visual function in Andhra Pradesh. Ophthalmology. 2007;114:1552–7.
Zebardast N, Swenor BK, van Landingham SW, Massof RW, Munoz B, West SK, et al. Comparing the Impact of Refractive and Nonrefractive Vision Loss on Functioning and Disability: The Salisbury Eye Evaluation. Ophthalmology. 2015;122:1102–10.
Oliver JE, Thulasiraj RD, Rahmathullah R, Baburajan, Katz J, Tielsch JM, et al. Vision-specific function and quality of life after cataract extraction in south India. J Cataract Refract Surg. 1998;24:222–9.
Finger RP, Kupitz DG, Fenwick E, Balasubramaniam B, Ramani RV, Holz FG, et al. The impact of successful cataract surgery on quality of life, household income and social status in South India. PLoS One. 2012;7:e44268.
Vignesh D, Gupta N, Kalaivani M, Goswami AK, Nongkynrih B, Gupta SK. Prevalence of visual impairment and its association with vision-related quality of life among elderly persons in a resettlement colony of Delhi. J Fam Med Prim care. 2019;8:1432–9.
Marmamula S, Kumbham TR, Modepalli SB, Chakrabarti S, Keeffe JE. Barriers to uptake of referral eye care services among the elderly in residential care: the Hyderabad Ocular Morbidity in Elderly Study (HOMES). Br J Ophthalmol. 2022;1:bjophthalmol-2021-320534.
Marmamula S, Kumbham TR, Modepalli SB, Chakrabarti S, Keeffe JE. Barriers to uptake of referral eye care services among the elderly in residential care: the Hyderabad Ocular Morbidity in Elderly Study (HOMES). Br J Ophthalmol. 2022;1:bjophthalmol-2021-320534.
Marmamula S, Yanamala NK, Khanna RC. “Eyecare on call” - Extending the frontiers of care through home-based eye care - Concept and the protocol. Indian J Ophthalmol. 2020;68:2625–7.
Acknowledgements
The authors thank the study participants for their committed contribution, Mr. Rajesh Challa and Ms. Madhuri Bhakki for their assistance in data collection, Ms. Muni Rajya Lakshmi for her support in data management, and Prof. Jill E. Keeffe (L V Prasad Eye Institute) for her inputs on earlier versions of the manuscript. Authors thank Ms. Neha Hassija for their language inputs on earlier versions of the manuscript.
Funding
This work was supported by Wellcome Trust/DBT India Alliance Fellowship [IA/CPHE/14/1/501506] awarded to Dr. Srinivas Marmamula and Hyderabad Eye Research Foundation (HERF), India.
Author information
Authors and Affiliations
Contributions
Study concept and design: SM. Acquisition, analysis, or interpretation of data: SM, TRK, SBM, RY. Drafting of the manuscript: SM. Critical revision of the manuscript for important intellectual content: SM, RCK, DSF. Statistical analysis: SM
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Marmamula, S., Barrenkala, N.R., Kumbham, T.R. et al. Impact of an intervention for avoidable vision loss on visual function in the elderly–The Hyderabad Ocular Morbidity in Elderly Study (HOMES). Eye 37, 1725–1731 (2023). https://doi.org/10.1038/s41433-022-02229-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-022-02229-6
- Springer Nature Limited