Abstract
Background
This study aimed to explore the functions of ubiquitin-specific protease 5 (USP5) in the endothelial inflammation of Kawasaki disease (KD).
Methods
USP5 expression levels in HCAECs were examined after stimulation with TNFα or KD sera. The inflammatory cytokine expression level and nuclear factor κB (NF-κB) signaling activation proteins were also investigated in HCAECs by using USP5 overexpression/knockdown lentivirus as well as its small molecule inhibitor vialinin A.
Results
USP5 expression level is upregulated in HCAECs after stimulation with KD sera. Similarly, the USP5 expression level is also increased in a time- and dose-dependent manner upon TNFα stimulation in HCAECs. Moreover, USP5 sustains proinflammatory cytokine production and NF-κB signaling activation, whereas USP5 knockdown causes the proinflammatory cytokine levels to decrease and suppress NF-κB signaling activation. Notably, the USP5 inhibitor vialinin A can suppress the expression of inflammatory genes induced by TNFα and IL-1β in HCAECs.
Conclusions
Our study identified USP5 as a positive regulator of TNFα production and its downstream signaling activation during the inflammatory responses in HCAECs, and demonstrated that its inhibitor vialinin A might serve as a candidate drug for KD therapy to prevent the excessive production of proinflammatory cytokines.
Impact
-
USP5 is upregulated in human coronary artery endothelial cells (HCAECs) whether incubated with acute KD sera or TNFα in vitro.
-
USP5 promotes proinflammatory cytokine expression by sustaining NF-κB signaling activation in HCAECs.
-
The USP5 inhibitor vialinin A can suppress the expression levels of proinflammatory cytokines in HCAEC, thus providing a novel mechanism and intervention strategy in KD therapy.
Similar content being viewed by others
Introduction
Kawasaki disease (KD), first described as an acute, febrile, mucocutaneous lymph node syndrome in 1967,1 is the most common systemic vasculitis in children and infants. It is often recognized as the leading cause of acquired heart disease among children in developed countries,2 which primarily involves the coronary arteries, but vasculitis can occur at various sites throughout the body.3 According to a recent epidemiological survey in Suzhou, China, the number of KD has been increasing annually between 2006 and 2017, with 24.5% of cases defined as coronary artery lesions (CALs).4 Although the exact pathological mechanism of CALs remains obscure, many studies have shown that KD-CALs are closely related to the overexpression and secretion of proinflammatory cytokines, such as interleukin-1β (IL-1β), interleukin-8 (IL-8), and tumor necrosis factor alpha (TNFα).5 Furthermore, many studies have shown that inflammation of vascular endothelial cells (ECs) plays a pivotal role in the pathogenesis of CALs in KD,6,7,8 and it is thought that ECs, as a source of proinflammatory cytokines, are stimulated to express and secret a variety of inflammatory factors in pathological states. This, in turn, causes recruitment and infiltration of inflammatory cells, thus resulting in subsequent local damage of vascular wall and formation of coronary artery aneurysm (CAA).9 However, the mechanisms by which ECs produce proinflammatory factors under KD conditions remain largely unknown.
The ubiquitin-specific protease 5 (USP5) belongs to the ubiquitin-specific protease family and has been identified as a tumor promoter in several types of human cancer, including pancreatic cancer, melanoma, non-small-cell lung cancer, and hepatocellular carcinoma.10 Furthermore, USP5 positively regulates TNFα expression level in rat basophilic leukemia (RBL-2H3) cells and is essential for TNFα production.11 Another study has shown that USP5 is upregulated in macrophages upon infection with Salmonella typhimurium.12 Together, these evidences suggest that USP5 is involved in the inflammatory response. However, whether USP5 is an important factor in KD pathogenesis remains unknown and needs to be investigated.
In this study, we aimed to explore whether USP5 is involved in the pathogenesis of KD and how it affects the inflammatory immune response in human coronary artery endothelial cells (HCAECs). We determined that USP5 expression level is increased in HCAECs after stimulation with KD sera, and its expression level can be induced by TNFα. Mechanistically, the USP5 expression level is positively correlated with proinflammatory cytokine expression via promoting NF-κB activation and this effect can be reversed by USP5 inhibitor vialinin A. Therefore, USP5 might function as a positive regulator in TNFα-mediated immune responses in HCAEC, whereas vialinin A antagonizes TNFα-mediated signaling by targeting USP5, thereby providing a novel target and therapeutic strategy in KD.
Methods
Human samples
The study protocol was approved by the Ethical Committee of the Children’s Hospital of Soochow University and followed the Declaration of Helsinki. All KD patients and healthy children were included in the study between January and March 2019 after obtaining written informed consent from their parents. All patients diagnosed with KD met the 2017 American Heart Association (AHA) diagnostic criteria. The acute stage was at the time of the disease diagnosis and admission, and before the intravenous immune globulin (IVIG) treatment. The afebrile stage was at 3 days after the IVIG treatment, and at this time, the patients’ temperature had recovered to normal range. The healthy children underwent regular health examinations and had no infections. All sera samples were filtered and stored at −80 °C until use.
Cell culture
The embryonic kidney 293 T cells were cultured in Dulbecco’s modified Eagle’s medium (Hyclone, South Logan, UT) supplemented with 10% fetal bovine serum (FBS) and 1% streptomycin–penicillin at 37 °C in a humidified 5% CO2 incubator. Primary HCAECs (Sciencell, Carlsbad, CA) were obtained and cultured using an Endothelial Cell Medium (Sciencell) containing 1% endothelial cell growth supplement and 10% FBS. When HCAECs were 90% confluent, the medium was exchanged with endothelial cell medium overnight and then incubated with 10% sera from KD patients or healthy children. Subsequently, HCAECs were cultured in a 5% CO2 incubator for 6 h at 37 °C. Recombinant human TNFα (Peprotech, Rocky Hill, NJ, 300-02AA) was purchased from Multi-sciences Corporation. The USP5 inhibitor vialinlin A was purchased from the MedChem Express Corporation (Monmouth Junction, NJ). All experiments were repeated at least three times.
Real-time quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from HCAECs using TRIzol reagent (Invitrogen, Carlsbad, CA). One microgram of the total RNA was reverse-transcribed to cDNA with random primers using the Revert Aid First Strand cDNA Synthesis Kit (Thermo Scientific, Waltham, MA, K1622), followed by RT-qPCR. The 2−ΔΔCT method was used to evaluate the relative quantities of each amplified product in the samples. The results were analyzed from three independent experiments and shown as the mean ± standard deviation (s.d.). The primers used for USP5, IL-8, TNFα, and GAPDH are listed as follows (Genewiz, SuZhou, China):
USP5-F, 5′-GGGAAGTACGGCAGGTGTCTA-3′.
USP5-R, 5′-CCATCAGTCAGGTTGAGCCAC-3′.
IL-8-F, 5′-CTGCGCCAACACAGAAATTAT-3′.
IL-8-R, 5′-CATCTGGCAACCCTACAACAG-3′.
TNFα-F, 5′-TCCTTCAGACACCCTCAACC-3′.
TNFα-R, 5′-AGGCCCCAGTTTGAATTCTT-3′.
GAPDH-F, 5′-ACCCACTCCTCCACCTTTGA-3′.
GAPDH-R, 5′-CTGTTGCTGTAGCCAAATTCGT-3′.
Western blot analysis
Proteins were quantified using a BCA protein assay kit (Thermo Scientific), with 25 μg of total protein separated using 8% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA). The following antibodies were used: anti-USP5 (Santa Cruz, CA, 1:1000, sc-390943), anti-Flag (Sigma, St.Louis, MO, 1:1000, F1804), anti-NF-ƙB (p65) (CST, Danvers, MA, 1:1000, 8242), anti-Phospho-NF-κB (p65) (ser536) (CST, 1:1000, 3031), anti-IKBα (Ser32) (CST, 1:1000, 4812), anti-Phospho-IκBα (CST, 1:1000, 2859), anti-α-Tubulin (Proteintech, 1:5000, 66031-1-Ig), and anti-GAPDH (Santa Cruz, 1:5000, sc47724). Image J program (http://rsbweb.nih.gov/ij/download.html) was used for densitometric analysis of western blots.
Lentivirus and plasmid
The lentiviral packaging plasmids psPAX2 and pMD2G were gifts from Dr. Li Gen (Institute of Pediatric Research, Children’s Hospital of Soochow University, China). Then the empty plasmid (Flag-CON), USP5 overexpression plasmid (Flag-USP5) and the packaging plasmids were used to construct lentivirus. The shUSP5 plasmid was purchased from GeneChem (Shanghai, China). Lentivirus containing negative control plasmid (Sh-NC) and shRNA USP5 plasmid (Sh-USP5) were constructed using a pLKO.1 vector (Genechem). HCAECs were infected at 100 MOI of lentivirus with 0.75 µɡ/ml polybrene (Merck, Darmstadt, Germany). After infections, the HCAECs were cultured in a growth medium complemented with 0.6 µɡ/ml puromycin (Beyotime, Shanghai, China). All plasmids were confirmed by DNA sequencing. HEK293T cells were transfected with LongTrans (Ucallm, WuXi, China), and HCAECs were transfected with JetPEI reagents (Polyplus, Strasbourg, France) according to the manufacturers’ instructions.
Statistics
GraphPad Prism 7.0 software was used for all statistical analyses. Normally distributed variables are presented as mean ± s.d. and statistically analyzed using t test to compare two experimental groups or one-way analysis of variance followed by Duncan multiple range test for comparisons among more than two groups. Not normally distributed variables were summarized as medians and interquartile ranges and statistically assessed by Mann–Whitney U-test. Categorical variables were compared using by chi-square test for frequencies and proportions. The number of asterisks represents the degree of significance with respect to p values. Differences were regarded statistically significance at a p < 0.05.
Results
Characteristics of KD patients and healthy control children
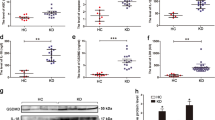
The demographic and clinical characteristics of KD patients were shown in Table 1. Forty-nine KD patients (25 boys and 24 girls) and 32 healthy children (HCs, 15 boys and 17 girls) were enrolled in the study. The median age of KD patients was 3.33 years (range 2.0–4.3). The duration of fever days was 5.0–8.0 days in KD patients. The acute KD subjects had higher values for white blood cells (WBCs), neutrophils% (N%), procalcitonin (PCT), high-sensitivity C reactive protein (hsCRP), TNFα, and IL-8 compared with the HCs (p < 0.05). The levels of WBCs, N%, PCT, and hsCRP were reduced at the afebrile stage. However, PLT was significantly higher than in the acute stage (p < 0.001). These results suggest that children with KD in the acute stage are in a state of inflammatory activation.
USP5 expression levels are increased in HCAECs incubated with KD sera or TNFα
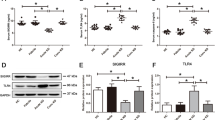
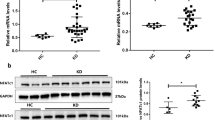
To investigate whether KD sera can cause USP5 expression level changes in HCAECs, we first examined the levels of USP5 mRNA and protein in HCAECs when treated with serum isolated from HCs (pooled from 32 individuals) or KD patients (pooled from 49 individuals) during either the acute or the afebrile stages. The mRNA and protein levels of USP5 in HCAECs after being treated with KD serum during the acute stage were higher than in those treated with the serum from HCs or patients during the afebrile phase (Fig. 1a, b). Consistently, the mRNA level of TNFα is also increased in HCAECs after being treated with serum isolated from acute KD patients (Fig. 1c). The USP5 expression level is positively correlated with the TNFα expression level during the acute phase in KD patients (Fig. 1d). Given that the proinflammatory stimuli TNFα plays an important role in KD pathogenesis, HCAECs were stimulated with TNFα to investigate the changes of USP5 expression level. Our results showed that both the mRNA and protein levels of USP5 were increased upon TNFα treatment in a dose- (Fig. 1e, f) and time-dependent (Fig. 1g, h) manner. Taken together, these data indicate that USP5 is upregulated in KD patients and might also be involved in the pathogenesis of HCAECs upon TNFα stimulation.
HCAECs were treated with medium containing 10% sera from HCs (pooled from 32 individuals), acute or afebrile KD (pooled from 49 individuals) subjects. After 6 h, protein level of USP5 (a) in HCAECs was measured by western blot, mRNA level of USP5 (b), and TNFα (c) were measured by RT-qPCR, the correlation (d) of the relative USP5 and TNFα levels was calculated using the GraphPad Prism Software in the KD acute group. Protein (e) and mRNA (f) levels of USP5 in HCAECs after the indicated doses of TNFα. Protein (g) and mRNA (h) levels of USP5 in HCAEC after TNFα treatment (10 ng/ml) at the indicated time. ns not significant; *p < 0.05, **p < 0.01, ***p < 0.001.
Overexpression and knockdown of USP5 by lentiviral vectors in HCAEC
Next, to investigate how USP5 is involved in the regulation of HCAECs, we constructed and used lentiviral vectors for USP5 that caused either an overexpression or a knockdown of USP5. An empty vector was used as a control. Lentiviral particles were produced in HEK293T cells. Briefly, lentiviral vectors encoding USP5 or ShUSP5 and packing mix were co-infected into HEK293T cells. Then, the supernatant of HEK293T cells was collected 48 h after lentiviral vector transfection and used to transfect HCAECs. The positively infected cells were selected with puromycin for 7 days (0.6 µg/ml) to obtained stable cell lines. Our results showed that USP5 was overexpressed in HCAECs, as verified by the mRNA and protein levels (Fig. 2a, b). The results indicate that the endogenous USP5 is successfully knocked down in HCAECs, as verified by the mRNA and protein levels, as well as fluorescence microscopy of green fluorescent protein (Fig. 2c–e).
HCAECs were infected with lentivirus containing empty plasmid (Flag-CON group) or Flag-USP5 expression plasmid (Flag-USP5 group) then selected with puromycin for 7 days (0.6 µg/ml) to obtained stable cell lines. Infection efficiency was confirmed at both mRNA (a) and protein (b) levels. HCAECs were infected with shRNA control (sh-NC group) or shUSP5(sh-USP5 group) lentivirus, the efficiency was confirmed at both mRNA (c) and protein (d) levels. e Fluorescence microscopy of HCAECs-GPF transfected with LV-shRNA-USP5 (MOI = 100). Scale bars, 200 µm. ns not significant; ***p < 0.001.
USP5 promotes IL-8 gene transcription in TNFα-treated HCAECs
Proinflammatory cytokine production in vascular endothelial cells is critical in the pathogenesis of KD. Thus, we tested the effect of USP5 on proinflammatory cytokine gene transcription in HCAECs with or without TNFα. We found that USP5 promotes gene transcription of cytokines such as IL-8 in a dose-dependent manner (Fig. 3a, b), whereas knockdown of USP5 has the opposite effects (Fig. 3c, d). The IL-8 mRNA was also enhanced in USP5-overexpressing HCAECs after TNFα treatment (Fig. 3e, f) and, conversely, the IL-8 gene transcription was suppressed in USP5-knockdown HCAECs (Fig. 3g, h).
HCAECs were transfected with control vector or different concentrations of Flag-tagged USP5 (0.5, 1.0 µg). After 24 h, cells were analyzed by RT-qPCR using specific primers for USP5 (a) and IL-8 (b). HCAECs were transfected with control (sh-NC) or shUSP5 plasmid (sh-USP5#1, sh-USP5#2) for 72 h. USP5 (c) and IL-8 (d) mRNA levels were evaluated by RT-qPCR. Flag-CON and Flag-USP5 HCAECs were treated with (blue) or without (red) TNFα (10 ng/ml) for 6 h and analyzed by RT-qPCR using specific primers for USP5 (e) and IL-8 (f). sh-NC and sh-USP5 HCAECs were treated with (blue) or without (red) TNFα (10 ng/ml) for 6 h and analyzed by RT-qPCR using specific primers for USP5 (g) and IL-8 (h). *p < 0.05, **p < 0.01, and ***p < 0.001.
USP5 promotes NF-κB signaling activation in TNFα-treated HCAECs
To further explore the regulatory mechanism by which USP5 promotes IL-8 gene transcription, we focused on NF-κB because it is a major intracellular regulator responsive to stimulation by proinflammatory cytokines.13 In addition, many studies have shown that NF-ƙB signaling is crucial for the pathological processes underlying KD, and especially so in the proinflammatory cytokine production in vascular endothelial cells.14 Therefore, we studied the effect of USP5 on TNFα-induced NF-ƙB activation. As shown in Fig. 4a, we found that phosphorylation of p65 (Fig. 4b) and IƙBα (Fig. 4c) were both increased in USP5-overexpressing HCAECs. In addition, the knockdown of the expression of USP5 suppressed phosphorylation of both p65 (Fig. 4d, e) and IƙBα (Fig. 4d, f), indicating that USP5 can promote NF-ƙB signaling activation.
a USP5-overexpressing HCAECs were stimulated with TNFα (10 ng/ml) for 15 min, followed by immunoblot assays with antibodies against the indicated proteins. The expression intensities a of USP5, p-p65, p65, pIƙBα, and IƙBα were quantified, and the ratios of p-P65/P65 (b) and pIƙBα/IƙBα (c) were calculated. USP5-knockdown HCAECs were stimulated with TNFα (10 ng/ml) for 15 min, followed by immunoblot assays with antibodies against the indicated proteins. The expression intensities d of USP5, p-p65, p65, pIƙBα, and IƙBα were quantified, and the ratios of p-P65/P65 (e) and pIƙBα/IƙBα (f) were calculated. *p < 0.05, **p < 0.01, and ***p < 0.001.
Effect of vialinin A on cytokine production in HCAECs
Previous studies have demonstrated that vialinin A inhibited (IC50 = 5.9 μM) the production and release of TNFα by strongly suppressing the enzymatic activity of USP5.11, 15 At concentrations below 5 μM, vialinin A did not have any effect on HUVEC cell viability at 24 and 48 h, whereas at a concentration of 10 μM HUVEC viability was decreased.16 Therefore, we used vialinin A at concentrations of 2.5 and 5 µM to examine its effect on the production of various inflammatory cytokines in HCAECs cultured with or without TNF-α/IL-1β. Vialinin A had an inhibitory effect on the mRNA expression for USP5 (Fig. 5a), IL-8 (Fig. 5c), and TNFα (Fig. 5d). Pretreatment with vialinin A also resulted in a significant decrease in the IL-1β-induced mRNA expression of USP5 (Fig. 5g), IL-8 (Fig. 5h), and TNFα (Fig. 5i), and preincubation with vialinin A dose-dependently prevented TNFα-induced mRNA expression of IL-8 (Fig. 5f). These results suggest that vialinin A could prevent inflammation by preventing the NF-ƙB-mediated expression of various inflammatory cytokines.
a Immunoblot analysis of USP5 expression and RT-qPCR analysis of USP5 (b), IL-8 (c), and TNFα (d) expression in HCAECs pretreated with vialinin A (2.5 µM) for 12 h. RT-qPCR analysis of USP5 (e) and IL-8 (f) expression in HCAECs pretreated with vialinin A (2.5 µM, 5.0 µM) for 12 h and followed by stimulation with TNFα (10 ng/ml) for 6 h. RT-qPCR analysis of USP5 (g), IL-8 (h), and TNFα (i) expression in HCAECs pretreated with vialinin A (2.5 µM) for 12 h and followed by treatment with IL-1β (20 ng/ml) for 6 h. M Mock, D DMSO, V vialinin A, ns not significant; ***p < 0.001.
Discussion
Here the expression level and functional roles of USP5 during the inflammatory process in KD and HCAECs were first investigated. Our results demonstrated that USP5 is upregulated in HCAECs, whether simulated with KD sera or TNFα in vitro, and USP5 can promote IL-8 expression by activating NF-κB signaling in HCAECs. Intriguingly, the USP5-induced overexpression of proinflammatory cytokines can be reversed by the USP5 inhibitor vialinin A. This previously unappreciated cellular mechanism sheds new light on the pathophysiology of KD and may open new avenues for the development of novel techniques with which to diagnose, evaluate, and treat this disease.
KD is an acute systemic vasculitis that involves inflammatory environment and endothelial damage. Innate and adaptive immune cells are activated, and inflammatory cytokines are released by a variety of cells, including ECs.8 TNFα has been well studied and implicated in the context of KD. Various studies have reported the clinical efficacy of blocking TNFα production in children resistant to standard IVIG and aspirin treatment,17 on the basis that USP5 is associated with TNFα production.11, 18 Here, to investigate that whether an upregulation of USP5 was linked to vascular endothelial damage in KD, the sera from KD patients were utilized to examine the effects of in vivo inflammatory environment on ECs. Our results showed that the USP5 expression level was upregulated in HCAECs after treated with KD sera, and its expression level was also positively correlated with the TNFα production during the acute phase of KD (Fig. 1). Taken together, these data indicate that USP5 is an important protease for regulating TNFα production in KD patients.
Furthermore, USP5 as an deubiquitinating enzyme can cleave different types of ubiquitin linkages.10 We previously reported that USP5 negatively regulates the IFN-I-induced signaling and antiviral activity by deubiquitinating SMURF1.19 Here we found that USP5 promotes IL-8 expression in TNFα-treated HCAECs (Fig. 3). Moreover, USP5 overexpression significantly promotes NF-κB signaling activation, whereas USP5 knockdown inhibits NF-κB signaling activation (Fig. 4). Future experiments using USP5−/− or transgenic mice will be helpful to reveal the mechanisms underlying its physiological and pathological roles in the regulation of NF-κB activation and inflammation in KD vasculitis.
Besides, USP5 could also slightly promote the NF-κB (Fig. 4a, g) and cytokine expression (Fig. 3b) even without TNFα stimulation. However, with the exogenous TNFα present, these phenomena have been significantly strengthened (Figs. 3f and 4a). We hypothesize that this effect may be related to the ability of HCAECs in resting state to secrete proinflammatory cytokines, such a low dose of cytokines could also promote USP5 expression and facilitate the interaction between USP5 and the unknown protein of NF-ƙB signaling pathway. Moreover, TNFα can induce the recruitment of many regulators to its downstream protein complex, such as A20, SPATA2, CYLD, LUBAC, and ANGPTL8, to keep the signaling finely tuned.20 This would be consistent with USP5 being crucial for the inflammatory processes in coronary artery endothelial cells in KD.
Importantly, our study also uncovered that vialinin A not only inhibits the expression of inflammatory factors induced by TNFα but also those induced by IL-1β in HCAECs (Fig. 5). Vialinin A is a p-terphenyl compound with antioxidant properties that was isolated from edible Chinese mushrooms Thelephora vialis21 and Thelephora terrestris.22 Besides the HCAECs, a previous study reported that vialinin A can also inhibit TNFα production in RBL-2H3 cells,11 which is consistent with our results. In fact, TNFα play essential roles in driving cardiac inflammation and promote acute myocarditis progression to coronary vasculitis23, 24 in KD patients and animal models of this disease. Therefore, the USP5-mediated regulation of cytokine expression levels in HCAECs can be reversed by its inhibitor vialinin A, thus providing a novel therapeutic strategy in KD children.
Conclusions
We determined that USP5 functions as a positive regulator of TNFα production and its downstream signaling activation during the inflammatory responses in HCAECs and that the USP5 inhibitor vialinin A might serve as a candidate intervention drug for KD therapy to prevent the excessive production of proinflammatory cytokines.
Data availability
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
References
Cohen, E. & Sundel, R. Kawasaki Disease at 50 Years. JAMA Pediatr. 170, 1093–1099 (2016).
McCrindle, B. W. et al. Diagnosis, treatment, and Long-Term management of kawasaki disease: A scientific statement for health professionals from the american heart association. Circulation. 135, (2017).
Takahashi, K., Oharaseki, T., Yokouchi, Y., Hiruta, N. & Naoe, S. Kawasaki disease as a systemic vasculitis in childhood. Ann. Vasc. Dis. 3, 173–181 (2010).
Sun, L. et al. Changes in profiles of kawasaki disease noted over time in suzhou, china. Cardiology 141, 25–31 (2018).
Noval, R. M. & Arditi, M. Kawasaki disease: Pathophysiology and insights from mouse models. Nat. Rev. Rheumatol. 16, 391–405 (2020).
Wang, Y. et al. The role of Ca2+/NFAT in Dysfunction and Inflammation of Human Coronary Endothelial Cells induced by Sera from patients with Kawasaki disease. Sci. Rep.-UK. 10, (2020).
Ueno, K. et al. Disruption of endothelial cell homeostasis plays a key role in the early pathogenesis of coronary artery abnormalities in kawasaki disease. Sci. Rep.-UK. 7, (2017).
Jia, C. et al. Endothelial cell pyroptosis plays an important role in Kawasaki disease via HMGB1/RAGE/cathespin B signaling pathway and NLRP3 inflammasome activation. Cell Death Dis. 10, (2019).
Huang, J., Wu, S., Cao, S., Zhu, X. & Zhang, S. Neutrophil-Derived semaphorin 4D induces inflammatory cytokine production of endothelial cells via different plexin receptors in kawasaki disease. Biomed. Res. Int. 2020, 1–11 (2020).
Ning, F. et al. Structure and function of USP5: Insight into physiological and pathophysiological roles. Pharmacol. Res. 157, 104557 (2020).
Yoshioka, Y. et al. Ubiquitin-specific peptidase 5, a target molecule of vialinin a, is a key molecule of TNF-alpha production in RBL-2H3 cells. PLoS One 8, e80931 (2013).
Kummari, E. et al. Activity-Based proteomic profiling of deubiquitinating enzymes in Salmonella-Infected macrophages leads to identification of putative function of UCH-L5 in inflammasome regulation. PLoS One 10, e0135531 (2015).
Aksentijevich, I. & Zhou, Q. NF-ƙB pathway in autoinflammatory diseases: Dysregulation of protein modifications by ubiquitin defines a new category of autoinflammatory diseases. Front. Immunol. 8, 399 (2017).
Ichiyama, T. et al. NF-κB activation in peripheral blood Monocytes/Macrophages and T cells during acute kawasaki disease. Clin. Immunol. 99, 373–377 (2001).
Onose, J. et al. Vialinin a, a novel potent inhibitor of TNF-alpha production from RBL-2H3 cells. Biol. Pharm. Bull. 31, 831–833 (2008).
Sonowal, H., Shukla, K., Kota, S., Saxena, A. & Ramana, K. V. Vialinin A, an edible Mushroom-Derived p-Terphenyl antioxidant, prevents VEGF-Induced neovascularization in vitro and in vivo. Oxid. Med. Cell. Longev. 2018, 1–10 (2018).
Yamaji, N. et al. TNF-alpha blockers for the treatment of Kawasaki disease in children. Cochrane Database Syst. Rev. 8, CD012448 (2019).
Onose, J. et al. Inhibitory effects of vialinin a and its analog on tumor necrosis factor-α release and production from RBL-2H3 cells. Cell. Immunol. 279, 140–144 (2012).
Qian, G. et al. Ubiquitin specific protease 5 negatively regulates the IFNs-mediated antiviral activity via targeting SMURF1. Int. Immunopharmacol. 87, 106763 (2020).
Zhou, Q. et al. USP15 potentiates NF-ƙB activation by differentially stabilizing TAB2 and TAB3. Febs. J. 287, 3165–3183 (2020).
Xie, C. et al. Vialinin A, a novel 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenger from an edible mushroom in China. Biosci. Biotechnol. Biochem. 69, 2326–2332 (2005).
Radulović, N., Quang, D. N., Hashimoto, T., Nukada, M. & Asakawa, Y. Terrestrins A–G: p-Terphenyl derivatives from the inedible mushroom Thelephora terrestris. Phytochemistry 66, 1052–1059 (2005).
Fujimaru, T. et al. Decreased levels of inflammatory cytokines in immunoglobulin-resistant Kawasaki disease after plasma exchange. Cytokine 70, 156–160 (2014).
Stock, A. T., Jama, H. A., Hansen, J. A. & Wicks, I. P. TNF and IL-1 play essential but temporally distinct roles in driving cardiac inflammation in a murine model of kawasaki disease. J. Immunol. 202, 3151–3160 (2019).
Acknowledgements
The authors would like to express their gratitude to EditSprings (https://www.editsprings.cn) for the expert linguistic services provided.
Funding
This project was funded by the National Natural Science Foundation of China (No. 82171797, No. 81971477, No. 81870365, No. 82070512, No. 81970436, No. 81900450), Jiangsu Provincial Social Development Project (SBE2021750252), Jiangsu Provincial Medical Young Talents (QNRC2016756), the Applied Foundational Research of Medical and Health Care of Suzhou City (SYS2019086, SYS2019083, KJXW2018021), Gusu Health Talent Program (GSWS2020038), and Natural Science Foundation for Youth of Wannan Medical College (WK2021F64).
Author information
Authors and Affiliations
Contributions
H.L. and G.Q. conceived the study and drafted the manuscript. C.H., W.W., H.H., J.J., Y.D., X.L., M.H., J.M., and X.P. conducted the experiments, analyzed the data, and participated in the discussion. All authors read and approved the final draft.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
Written informed consent was obtained from all participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, C., Wang, W., Huang, H. et al. Kawasaki disease: ubiquitin-specific protease 5 promotes endothelial inflammation via TNFα-mediated signaling. Pediatr Res 93, 1883–1890 (2023). https://doi.org/10.1038/s41390-022-02341-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41390-022-02341-z
- Springer Nature America, Inc.