Abstract
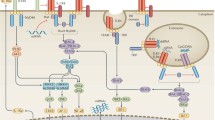
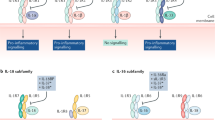
Toll-like receptors (TLRs) have caught the attention of rheumatologists searching for additional therapeutic targets for diseases such as rheumatoid arthritis and systemic lupus erythematosus. Signaling from these molecules can induce the expression of cytokines such as tumor necrosis factor and interferon α. Strategies that target TLRs and their co-receptors (such as MD2 for TLR4 or CD36 for TLR2) might be a more-selective approach than inhibition of global signals such as nuclear factor κB or p38 mitogen-activated protein kinase. TLR signaling requires adaptor proteins, including MyD88, Mal, TRIF and TRAM, which are recruited to specific receptors: Mal is used only by TLR2 and TLR4, TRIF is used by TLR3 and TLR4, and TRAM is recruited by TLR4 alone. Mal and TRAM are subject to complex biochemical regulation. Inhibition of Mal or MyD88 blocks the production of inflammatory mediators in synovial tissue. Another possible intracellular target is Unc93b, a protein involved in signaling from TLR3, TLR7 and TLR9. Inhibition of TLR4, TLR7 and TLR9 has produced intriguing results, which indicate that TLRs and their signaling pathways might indeed have great potential as novel targets for the treatment of inflammatory joint disease.
Key Points
-
Toll-like receptors (TLRs) are important innate immune receptors that trigger inflammatory signaling pathways in response to microbial products or the products of inflamed tissues
-
TLRs are expressed in inflamed joints and are strong inducers of inflammatory cytokines
-
Inhibition of certain TLRs has resulted in anti-inflammatory and joint-protective effects in animal models of arthritis
-
TLRs activate complex signaling pathways that include specific adaptor proteins
-
Highly complex biochemical and genetic regulation of the adaptor proteins has been uncovered, and thereby presents possible new targets for use in the effort to limit the inflammatory effects of TLRs



Similar content being viewed by others
References
O'Neill LA (2005) Immunity's early warning system. Sci Am 292: 24–31
Sacre SM et al. (2007) Could Toll-like receptors provide a missing link in chronic inflammation in rheumatoid arthritis? Lessons from a study on human rheumatoid tissue. Ann Rheum Dis 66 (Suppl 3): 81–86
Müller-Ladner U et al. (2005) Mechanisms of disease: the molecular and cellular basis of joint destruction in rheumatoid arthritis. Nat Clin Pract Rheumatol 1: 102–110
Lenert PS et al. (2006) Targeting Toll-like receptor signaling in plasmacytoid dendritic cells and autoreactive B cells as a therapy for lupus. Arthritis Res Ther 8: 203
Barrat FJ et al. (2005) Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J Exp Med 202: 1131–1139
Sheedy F and O'Neill LA (2007) The Troll in Toll: Mal and TRAM as bridges for TLR2 and TLR4 signaling. J Leukoc Biol 82: 196–203
O'Neill LA and Bowie AG (2007) The family of five: TIR-domain-containing adaptors in TLR signalling. Nat Rev Immunol 7: 353–364
Kanzler H et al. (2007) Therapeutic targeting of innate immunity with Toll-like receptor agonists and antagonists. Nat Med 13: 552–559
Schaefer L et al. (2005) The matrix component biglycan is proinflammatory and signals via Toll-like receptors 4 and 2 in macrophages. J Clin Invest 115: 2223–2233
Termeer C et al. (2002) Oligosaccharides of hyaluronan activate dendritic cells via Toll-like receptor-4. J Exp Med 195: 99–111
Roelofs MF et al. (2006) Identification of small heat shock protein B8 (HSP22) as a novel TLR4 ligand and potential involvement in the pathogenesis of rheumatoid arthritis. J Immunol 176: 7021–7027
Park JS et al. (2006) High mobility group box 1 protein interacts with multiple Toll-like receptors. Am J Physiol Cell Physiol 290: C917–C924
Johnson GB et al. (2002) Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J Immunol 168: 5233–5239
Okamura Y et al. (2001) The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem 276: 10229–10233
Smiley ST et al. (2001) Fibrinogen stimulates macrophage chemokine secretion through Toll-like receptor 4. J Immunol 167: 2887–2894
Brentano F et al. (2005) RNA released from necrotic synovial fluid cells activates rheumatoid arthritis synovial fibroblasts via Toll-like receptor 3. Arthritis Rheum 52: 2656–2665
Vollmer J et al. (2005) Immune stimulation mediated by autoantigen binding sites within small nuclear RNAs involves Toll-like receptors 7 and 8. J Exp Med 202: 1575–1585
Leadbetter EA et al. (2002) Chromatin–IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature 416: 603–607
Pierer M et al. (2004) Chemokine secretion of rheumatoid arthritis synovial fibroblasts stimulated by Toll-like receptor 2 ligands. J Immunol 172: 1256–1265
Jin MS et al. (2007) Crystal structure of the TLR1–TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell 130: 1071–1082
Núñez Miguel R et al. (2007) A dimer of Toll-like receptor 4 cytoplasmic domain provides a specific scaffold for the recruitment of signalling adaptor proteins. PLoS ONE 2: e788
Ohto U et al. (2007) Crystal structure of human MD2 and its complex with antiendotoxic lipid IVa. Science 316: 1632–1634
Hoebe K et al. (2005) CD36 is a sensor of diacylglycerides. Nature 433: 523–527
Barral DC and Brenner MB (2007) CD1 antigen presentation: how it works. Nat Rev Immunol 7: 929–941
Mansell A et al. (2006) Suppressor of cytokine signaling 1 negatively regulates Toll-like receptor signaling by mediating Mal degradation. Nat Immunol 7: 148–155
Gray P et al. (2006) MyD88 adapter-like (Mal) is phosphorylated by Bruton's tyrosine kinase during TLR2 and TLR4 signal transduction. J Biol Chem 281: 10489–10495
Miggin S et al. (2007) NF-κB activation by the Toll-IL-1 receptor domain-containing protein Mal is regulated by caspase-1. Proc Natl Acad Sci USA 104: 3372–3377
Kagan JC and Medzhitov R (2006) Phosphoinositide-mediated adapter recruitment controls Toll-like receptor signaling. Cell 125: 943–955
Mansell A et al. (2004) Mal interacts with tumor necrosis factor receptor-associated factor (TRAF)-6 to mediate NF-κB activation by TLR2 and TLR4. J Biol Chem 279: 37227–37230
Khor CC et al. (2007) A Mal functional variant is associated with protection against invasive pneumococcal disease, bacteremia, malaria and tuberculosis. Nat Genet 39: 523–528
Rowe D et al. (2006) The myristoylation of TRIF-related adaptor molecule is essential for Toll-like receptor 4 signal transduction. Proc Natl Acad Sci USA 103: 6299–6304
McGettrick A et al. (2006) Trif-related adapter molecule is phosphorylated by protein kinase C epsilon during Toll-like receptor 4 signaling. Proc Natl Acad Sci USA 103: 9196–9201
Tabeta K et al. (2006) The Unc93b1 mutation 3d disrupts exogenous antigen presentation and signaling via Toll-like receptors 3, 7 and 9. Nat Immunol 7: 156–164
Brinkmann MM et al. (2007) The interaction between the ER membrane protein UNC93B and TLR3, 7 and 9 is crucial for TLR signaling. J Cell Biol 177: 265–275
de Bouteiller O et al. (2005) Recognition of double-stranded RNA by human Toll-like receptor 3 and downstream receptor signaling requires multimerisation and acidic pH. J Biol Chem 280: 38133–38145
Kyburz D et al. (2006) Mode of action of hydroxychloroquine in RA—evidence of an inhibitory effect on Toll-like receptor signaling. Nat Clin Pract Rheumatol 2: 458–459
Abdollahi-Roodsaz S et al. (2008) Stimulation of TLR2 and TLR4 differentially skews the balance of T cells in a mouse model of arthritis. J Clin Invest 118: 1–13
Abdollahi-Roodsaz S et al. (2007) Inhibition of Toll-like receptor 4 breaks the inflammatory loop in autoimmune destructive arthritis. Arthritis Rheum 56: 2957–2967
Hegen M et al. (2007) Utility of animal models for identification of potential therapeutics for rheumatoid arthritis. Ann Rheum Dis [10.1136/ard.2007.076430]
Popa C et al. (2007) Bartonella quintana lipopolysaccharide is a natural antagonist of Toll-like receptor 4. Infect Immun 75: 4831–4837
Sacre SM et al. (2007) The Toll-like receptor adapter proteins MyD88 and Mal/TIRAP contribute to the inflammatory and destructive processes in a human model of rheumatoid arthritis. Am J Pathol 170: 518–525
Barrat FJ et al. (2007) Treatment of lupus-prone mice with a dual inhibitor of TLR7 and TLR9 leads to reduction of autoantibody production and amelioration of disease symptoms. Eur J Immunol 37: 3582–3586
Ogawa S et al. (2005) Molecular determinants of crosstalk between nuclear receptors and Toll-like receptors. Cell 122: 707–721
Vanags D et al. (2006) Therapeutic efficacy and safety of chaperonin 10 in patients with rheumatoid arthritis: a double-blind randomised trial. Lancet 368: 855–863
Toshchakov V et al. (2005) Differential involvement of BB loops of Toll-IL-1 resistance (TIR) domain-containing adapter proteins in TLR4- versus TLR2-mediated signal transduction. J Immunol 175: 494–500
Bartfai T et al. (2003) A low molecular weight mimic of the Toll/IL-1 receptor/resistance domain inhibits IL-1 receptor-mediated responses. Proc Natl Acad Sci USA 100: 7971–7976
Low W et al. (2007) Functional cell permeable motifs within medically relevant peptides. J Biotech 129: 555–564
Davis CN et al. (2006) MyD88-dependent and -independent signaling by IL-1 in neurons by bifunctional Toll/IL-1 receptor domain/BB loop mimetics. Proc Natl Acad Sci USA 103: 2953–2958
Carty M et al. (2006) The human adaptor SARM negatively regulates adaptor protein TRIF-dependent Toll-like receptor signaling. Nat Immunol 7: 1074–1081
Marshak-Rothstein A (2006) Toll-like receptors in systemic lupus erythematosus. Nat Rev Immunol 6: 823–835
Rothlin CV et al. (2007) TAM receptors are pleiotropic inhibitors of the innate immune response. Cell 131: 1124–1136
Acknowledgements
The author would like to thank the Science Foundation Ireland and the Irish Health Research Board for research funding.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
O'Neill, L. Primer: Toll-like receptor signaling pathways—what do rheumatologists need to know?. Nat Rev Rheumatol 4, 319–327 (2008). https://doi.org/10.1038/ncprheum0802
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncprheum0802
- Springer Nature Limited
This article is cited by
-
Propofol Attenuates Airway Inflammation in a Mast Cell-Dependent Mouse Model of Allergic Asthma by Inhibiting the Toll-like Receptor 4/Reactive Oxygen Species/Nuclear Factor κB Signaling Pathway
Inflammation (2018)
-
N-(2-hydroxy phenyl) acetamide: a novel suppressor of Toll-like receptors (TLR-2 and TLR-4) in adjuvant-induced arthritic rats
Molecular and Cellular Biochemistry (2014)
-
Current and emerging therapeutic strategies for Fanconi anemia
The HUGO Journal (2012)
-
NADPH oxidase-mediated redox signal contributes to lipoteichoic acid-induced MMP-9 upregulation in brain astrocytes
Journal of Neuroinflammation (2012)
-
Upregulation of tumor necrosis factor receptor-associated factor 6 correlated with synovitis severity in rheumatoid arthritis
Arthritis Research & Therapy (2012)