Abstract
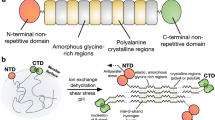
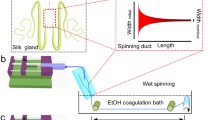
Herein we present a chimeric recombinant spider silk protein (spidroin) whose aqueous solubility equals that of native spider silk dope and a spinning device that is based solely on aqueous buffers, shear forces and lowered pH. The process recapitulates the complex molecular mechanisms that dictate native spider silk spinning and is highly efficient; spidroin from one liter of bacterial shake-flask culture is enough to spin a kilometer of the hitherto toughest as-spun artificial spider silk fiber.


Similar content being viewed by others
References
Askarieh, G. et al. Nature 465, 236–238 (2010).
Hagn, F. et al. Nature 465, 239–242 (2010).
Zhang, H. et al. Prep. Biochem. Biotechnol. 46, 552–558 (2016).
Copeland, C.G., Bell, B.E., Christensen, C.D. & Lewis, R.V. ACS Biomater. Sci. Eng. 1, 577–584 (2015).
Lin, Z., Deng, Q., Liu, X.Y. & Yang, D. Adv. Mater. 25, 1216–1220 (2013).
Adrianos, S.L. et al. Biomacromolecules 14, 1751–1760 (2013).
Xu, L., Rainey, J.K., Meng, Q. & Liu, X.Q. PLoS One 7, e50227 (2012).
Albertson, A.E., Teulé, F., Weber, W., Yarger, J.L. & Lewis, R.V. J. Mech. Behav. Biomed. Mater. 29, 225–234 (2014).
Xia, X.X. et al. Proc. Natl. Acad. Sci. USA 107, 14059–14063 (2010).
Teulé, F., Furin, W.A., Cooper, A.R., Duncan, J.R. & Lewis, R.V. J. Mater. Sci. 42, 8974–8985 (2007).
Stark, M. et al. Biomacromolecules 8, 1695–1701 (2007).
Rammensee, S., Slotta, U., Scheibel, T. & Bausch, A.R. Proc. Natl. Acad. Sci. USA 105, 6590–6595 (2008).
Heidebrecht, A. et al. Adv. Mater. 27, 2189–2194 (2015).
Hijirida, D.H. et al. Biophys. J. 71, 3442–3447 (1996).
Andersson, M. et al. PLoS Biol. 12, e1001921 (2014).
Kronqvist, N. et al. Nat. Commun. 5, 3254 (2014).
Gaines, W.A., Sehorn, M.G. & Marcotte, W.R. Jr. J. Biol. Chem. 285, 40745–40753 (2010).
Rising, A. & Johansson, J. Nat. Chem. Biol. 11, 309–315 (2015).
Gauthier, M., Leclerc, J., Lefèvre, T., Gagné, S.M. & Auger, M. Biomacromolecules 15, 4447–4454 (2014).
Vollrath, F. & Knight, D.P. Int. J. Biol. Macromol. 24, 243–249 (1999).
Giesa, T., Perry, C.C. & Buehler, M.J. Biomacromolecules 17, 427–436 (2016).
Lin, Z., Huang, W., Zhang, J., Fan, J.S. & Yang, D. Proc. Natl. Acad. Sci. USA 106, 8906–8911 (2009).
Shen, C.L. & Murphy, R.M. Biophys. J. 69, 640–651 (1995).
Lefèvre, T., Boudreault, S., Cloutier, C. & Pézolet, M. Biomacromolecules 9, 2399–2407 (2008).
Jiang, P. et al. Sci. Rep. 4, 7326 (2014).
Porter, D., Guan, J. & Vollrath, F. Adv. Mater. 25, 1275–1279 (2013).
Rising, A., Hjälm, G., Engström, W. & Johansson, J. Biomacromolecules 7, 3120–3124 (2006).
Lefèvre, T., Rousseau, M.E. & Pézolet, M. Biophys. J. 92, 2885–2895 (2007).
Huang, W. et al. Macromolecules 47, 8107–8114 (2014).
Ling, S., Qi, Z., Knight, D.P., Shao, Z. & Chen, X. Biomacromolecules 12, 3344–3349 (2011).
Larson, J.L., Ko, E. & Miranker, A.D. Protein Sci. 9, 427–431 (2000).
Strohalm, M., Kavan, D., Novák, P., Volný, M. & Havlícek, V. Anal. Chem. 82, 4648–4651 (2010).
Acknowledgements
We thank L. Holm, the Swedish University of Agricultural Sciences for help with photography, as well as S. Takeuchi and A. Hsiao at the University of Tokyo for introduction into the use of pulled glass capillaries for fiber formation. We also thank F. Palm, Uppsala University, for lending us a microelectrode puller. Q.J. was supported by a stipend from the Chinese Scholarship Council. The Swedish Research Council (grants no. 2014-2408 and 2014-10371 to A.R. and J.J.), CIMED (to J.J.) and FORMAS (2015-629 to A.R.) supported this work.
Author information
Authors and Affiliations
Contributions
M.A., Q.J., A.A., X.-Y.L., M.L., and P.P. performed the experiments; A.R., J.J., G.R.P., Q.M., C.V.R., M.T., H.H. supplied equipment and expertise; A.R. and J.J. conceived and designed the study; M.A., A.R. and J.J. wrote the manuscript. All authors edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Results, Supplementary Tables 1 and 2 and Supplementary Figures 1–7. (PDF 819 kb)
Supplementary Table
Source data for Supplementary Figure 7. (XLSX 135 kb)
41589_2017_BFnchembio2269_MOESM590_ESM.mov
Spinning NT2RepCT in a biomimetic spinning device. Fibers form instantaneously as the highly concentrated spinning dope hits the pH 5.0 aqueous buffer. (MOV 23001 kb)
Source data
Rights and permissions
About this article
Cite this article
Andersson, M., Jia, Q., Abella, A. et al. Biomimetic spinning of artificial spider silk from a chimeric minispidroin. Nat Chem Biol 13, 262–264 (2017). https://doi.org/10.1038/nchembio.2269
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nchembio.2269
- Springer Nature America, Inc.
This article is cited by
-
Replicating shear-mediated self-assembly of spider silk through microfluidics
Nature Communications (2024)
-
Bioinspired and biomimetic protein-based fibers and their applications
Communications Materials (2024)
-
Bi-terminal fusion of intrinsically-disordered mussel foot protein fragments boosts mechanical strength for protein fibers
Nature Communications (2023)
-
Regionalization of cell types in silk glands of Larinioides sclopetarius suggest that spider silk fibers are complex layered structures
Scientific Reports (2023)
-
Electrospun Flexible Nanofibres for Batteries: Design and Application
Electrochemical Energy Reviews (2023)