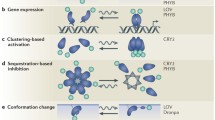
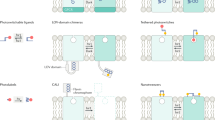
Abstract
In the study of complex mammalian behaviours, technological limitations have prevented spatiotemporally precise control over intracellular signalling processes. Here we report the development of a versatile family of genetically encoded optical tools (‘optoXRs’) that leverage common structure–function relationships1 among G-protein-coupled receptors (GPCRs) to recruit and control, with high spatiotemporal precision, receptor-initiated biochemical signalling pathways. In particular, we have developed and characterized two optoXRs that selectively recruit distinct, targeted signalling pathways in response to light. The two optoXRs exerted opposing effects on spike firing in nucleus accumbens in vivo, and precisely timed optoXR photostimulation in nucleus accumbens by itself sufficed to drive conditioned place preference in freely moving mice. The optoXR approach allows testing of hypotheses regarding the causal impact of biochemical signalling in behaving mammals, in a targetable and temporally precise manner.




Similar content being viewed by others
References
Karnik, S. S. et al. Activation of G-protein-coupled receptors: A common molecular mechanism. Trends Endocrinol. Metab. 14, 431–437 (2003)
Kim, J. M. et al. Light-driven activation of β2-adrenergic receptor signaling by a chimeric rhodopsin containing the β2-adrenergic receptor cytoplasmic loops. Biochemistry 44, 2284–2292 (2005)
Pierce, K. L., Premont, R. T. & Lefkowitz, R. J. Seven-transmembrane receptors. Nature Rev. Mol. Cell Biol. 3, 639–650 (2002)
Zhang, F. et al. Channelrhodopsin-2 and optical control of excitable cells. Nature Meth. 3, 785–792 (2006)
Oliveira, L., Paiva, A. C. M. & Vriend, G. A low resolution model for the interaction of G proteins with G protein-coupled receptors. Protein Eng. 12, 1087–1095 (1999)
Palczewski, K. G protein-coupled receptor rhodopsin. Annu. Rev. Biochem. 75, 743–767 (2006)
Azzi, M. et al. β-Arrestin-mediated activation of MAPK by inverse agonists reveals distinct active conformations for G protein-coupled receptors. Proc. Natl Acad. Sci. USA 100, 11406–11411 (2003)
Kenakin, T. Special issue on allosterism and collateral efficacy. Trends Pharmacol. Sci. 28, 359–446 (2007)
Shukla, A. K. et al. Distinct conformational changes in β-arrestin report biased agonism at seven-transmembrane receptors. Proc. Natl Acad. Sci. USA 105, 9988–9993 (2008)
Rich, T. C. et al. In vivo assessment of local phosphodiesterase activity using tailored cyclic nucleotide-gated channels as cAMP sensors. J. Gen. Physiol. 118, 63–78 (2001)
Wilson, C. J. in The Synaptic Organization of the Brain (ed. Shepherd, G.) 361–413 (Oxford Univ. Press, 2003)
Gradinaru, V. et al. Targeting and readout strategies for fast optical neural control in vitro and in vivo. J. Neurosci. 27, 14231–14238 (2007)
Deisseroth, K. et al. Signaling from synapse to nucleus: The logic behind the mechanisms. Curr. Opin. Neurobiol. 13, 354–365 (2003)
White, F. J. & Wang, R. Y. Electrophysiological evidence for the existence of both D-1 and D-2 dopamine receptors in the rat nucleus accumbens. J. Neurosci. 6, 274–280 (1986)
Hyman, S. E., Malenka, R. C. & Nestler, E. J. Neural mechanisms of addiction: The role of reward-related learning and memory. Annu. Rev. Neurosci. 29, 565–598 (2006)
Tobler, P. N., Fiorillo, C. D. & Schultz, W. Adaptive coding of reward value by dopamine neurons. Science 307, 1642–1645 (2005)
Potts, J. T. & Waldrop, T. G. Discharge patterns of somatosensitive neurons in the nucleus tractus solitarius of the cat. Neuroscience 132, 1123–1134 (2005)
Pettit, D. L. et al. Chemical two-photon uncaging: A novel approach to mapping glutamate receptors. Neuron 19, 465–471 (1997)
Furuta, T. et al. Brominated 7-hydroxycoumarin-4-ylmethyls: Photolabile protecting groups with biologically useful cross-sections for two photon photolysis. Proc. Natl Acad. Sci. USA 96, 1193–1200 (1999)
Conklin, B. R. et al. Engineering GPCR signaling pathways with RASSLs. Nature Meth. 5, 673–678 (2008)
Lima, S. Q. & Miesenböck, G. Remote control of behavior through genetically targeted photostimulation of neurons. Cell 121, 141–152 (2005)
Zemelman, B. V. et al. Selective photostimulation of genetically chARGed neurons. Neuron 33, 15–22 (2002)
Li, X. et al. Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc. Natl Acad. Sci. USA 102, 17816–17821 (2005)
Schroder-Lang, S. et al. Fast manipulation of cellular cAMP level by light in vivo. Nature Meth. 4, 39–42 (2007)
Cohen, G. B. et al. Constitutive activation of opsin: Influence of charge at position 134 and size at position 296. Biochemistry 32, 6111–6115 (1993)
Carelli, R. M. & Wightman, R. M. Functional microcircuitry in the accumbens underlying drug addiction: Insights from real-time signaling during behavior. Curr. Opin. Neurobiol. 14, 763–768 (2004)
Dunn, T. A. et al. Imaging of cAMP levels and protein kinase A activity reveals that retinal waves drive oscillations in second-messenger cascades. J. Neurosci. 26, 12807–12815 (2006)
Zhang, F. et al. Multimodal fast optical interrogation of neural circuitry. Nature 446, 633–639 (2007)
Petreanu, L. et al. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nature Neurosci. 10, 663–668 (2007)
Airan, R. D. et al. Integration of light-controlled neuronal firing and fast circuit imaging. Curr. Opin. Neurobiol. 17, 587–592 (2007)
Acknowledgements
We thank B. Kobilka, B. Knutson, M. P. Bokoch, T. Sudhof, R. Malenka and the Deisseroth Laboratory for comments and discussion. We appreciate the gifts of pCNGA2-C460W/E583M from J. W. Karpen, pcDNA3.1-β2AR from B. Kobilka and pDT-α1AR from C. Hague. We thank T. Jardetzky for use of a Biotek Synergy4 plate reader. R.D.A. is supported by a NIH/NIMH National Research Service Award and the Stanford Medical Scientist Training Program. K.R.T. is supported by a NARSAD Young Investigator Award. K.D. is supported by CIRM, McKnight, Coulter, Klingenstein, Keck, NSF, NIMH, NIDA, the NIH Pioneer Award, the Albert Yu and Mary Bechmann Foundation and the Kinetics Foundation.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
This file contains Supplementary Methods, Supplementary Figures S1-S4 with Legends, Supplementary Tables S1-S4 and Supplementary References (PDF 913 kb)
Rights and permissions
About this article
Cite this article
Airan, R., Thompson, K., Fenno, L. et al. Temporally precise in vivo control of intracellular signalling. Nature 458, 1025–1029 (2009). https://doi.org/10.1038/nature07926
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature07926
- Springer Nature Limited
This article is cited by
-
Neuronal and astrocytic CB1R signaling differentially modulates goal-directed behavior and working memory by distinct temporal mechanisms
Neuropsychopharmacology (2023)
-
Adrenergic signalling to astrocytes in anterior cingulate cortex contributes to pain-related aversive memory in rats
Communications Biology (2023)
-
Optimized design and in vivo application of optogenetically functionalized Drosophila dopamine receptors
Nature Communications (2023)
-
Multiple opsins in a reef-building coral, Acropora millepora
Scientific Reports (2023)
-
Polydimethylsiloxane as a more biocompatible alternative to glass in optogenetics
Scientific Reports (2023)