Abstract
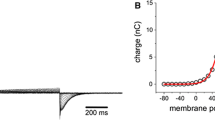
Voltage-gated ion channels are responsible for generating electrical impulses in nerves and other excitable cells. The fourth transmembrane helix (S4) in voltage-gated channels is the primary voltage-sensing unit that mediates the response to a changing membrane electric field1,2. The molecular mechanism of voltage sensing, particularly with respect to the magnitude of the transmembrane movement of S4, remains controversial3,4,5. To determine the extent of this transmembrane movement, we use fluorescent resonance energy transfer between the S4 domain and a reference point in the lipid bilayer. The lipophilic ion dipicrylamine distributes on either side of the lipid bilayer depending on the membrane potential, and is used here as a resonance-energy-transfer acceptor from donor molecules attached to several positions in the Shaker K+ channel. A voltage-driven transmembrane movement of the donor should produce a transient fluorescence change because the acceptor also translocates as a function of voltage. In Shaker K+ channels no such transient fluorescence is observed, indicating that the S4 segment does not translocate across the lipid bilayer. Based on these observations, we propose a molecular model of voltage gating that can account for the observed 13e gating charge with limited transmembrane S4 movement.



Similar content being viewed by others
References
Aggarwal, S. K. & MacKinnon, R. Contribution of the S4 segment to gating charge in the Shaker K+ channel. Neuron 16, 1169–1177 (1996)
Seoh, S. A., Sigg, D., Papazian, D. M. & Bezanilla, F. Voltage-sensing residues in the S2 and S4 segments of the Shaker K+ channel. Neuron 16, 1159–1167 (1996)
Bezanilla, F. The voltage sensor in voltage-dependent ion channels. Physiol. Rev. 80, 555–592 (2000)
Ahern, C. A. & Horn, R. Stirring up controversy with a voltage sensor paddle. Trends Neurosci. 27, 303–307 (2004)
Jiang, Y., Ruta, V., Chen, J., Lee, A. & MacKinnon, R. The principle of gating charge movement in a voltage-dependent K(+ ) channel. Nature 423, 42–48 (2003)
Guy, H. R. & Seetharamulu, P. Molecular model of the action potential sodium channel. Proc. Natl Acad. Sci. USA 83, 508–512 (1986)
Catterall, W. A. Molecular properties of voltage-sensitive sodium channels. Annu. Rev. Biochem. 55, 953–985 (1986)
Jiang, Y. et al. X-ray structure of a voltage-dependent K(+ ) channel. Nature 423, 33–41 (2003)
Yang, N., George, A. L. Jr & Horn, R. Molecular basis of charge movement in voltage-gated sodium channels. Neuron 16, 113–122 (1996)
Larsson, H. P., Baker, O. S., Dhillon, D. S. & Isacoff, E. Y. Transmembrane movement of the shaker K+ channel S4. Neuron 16, 387–397 (1996)
Starace, D. M., Stefani, E. & Bezanilla, F. Voltage-dependent proton transport by the voltage sensor of the Shaker K+ channel. Neuron 19, 1319–1327 (1997)
Starace, D. M. & Bezanilla, F. A proton pore in a potassium channel voltage sensor reveals a focused electric field. Nature 427, 548–553 (2004)
Cha, A., Snyder, G. E., Selvin, P. R. & Bezanilla, F. Atomic scale movement of the voltage-sensing region in a potassium channel measured via spectroscopy. Nature 402, 809–813 (1999)
Glauner, K. S., Mannuzzu, L. M., Gandhi, C. S. & Isacoff, E. Y. Spectroscopic mapping of voltage sensor movement in the Shaker potassium channel. Nature 402, 813–817 (1999)
Asamoah, O. K., Wuskell, J. P., Loew, L. M. & Bezanilla, F. A fluorometric approach to local electric field measurements in a voltage-gated ion channel. Neuron 37, 85–97 (2003)
Gonzalez, J. E. & Tsien, R. Y. Voltage sensing by fluorescence resonance energy transfer in single cells. Biophys. J. 69, 1272–1280 (1995)
Tosteson, M. T. & Tosteson, D. C. The sting. Melittin forms channels in lipid bilayers. Biophys. J. 36, 109–116 (1981)
Kempf, C. et al. Voltage-dependent trans-bilayer orientation of melittin. J. Biol. Chem. 257, 2469–2476 (1982)
Laine, M. et al. Atomic proximity between S4 segment and pore domain in Shaker potassium channels. Neuron 39, 467–481 (2003)
Islas, L. D. & Sigworth, F. J. Electrostatics and the gating pore of Shaker potassium channels. J. Gen. Physiol. 117, 69–89 (2001)
Roux, B. Influence of the membrane potential on the free energy of an intrinsic protein. Biophys. J. 73, 2980–2989 (1997)
Abramson, J. et al. Structure and mechanism of the lactose permease of Escherichia coli. Science 301, 610–615 (2003)
Boland, L. M., Jurman, M. E. & Yellen, G. Cysteines in the Shaker K+ channel are not essential for channel activity or zinc modulation. Biophys. J. 66, 694–699 (1994)
Chanda, B. & Bezanilla, F. Tracking voltage-dependent conformational changes in skeletal muscle sodium channel during activation. J. Gen. Physiol. 120, 629–645 (2002)
Cha, A. & Bezanilla, F. Structural implications of fluorescence quenching in the Shaker K+ channel. J. Gen. Physiol. 112, 391–408 (1998)
Zhou, Y., Morais-Cabral, J. H., Kaufman, A. & MacKinnon, R. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0Å resolution. Nature 414, 43–48 (2001)
Gonzalez, C., Rosenman, E., Bezanilla, F., Alvarez, O. & Latorre, R. Periodic perturbations in Shaker K+ channel gating kinetics by deletions in the S3–S4 linker. Proc. Natl Acad. Sci. USA 98, 9617–9623 (2001)
Brooks, B. R. et al. CHARMM: A program for macromolecular energy, minimization, and dynamics calculations. J. Comput. Chem. 4, 187–217 (1983)
Nina, M., Beglov, D. & Roux, B. Atomic Born radii for continuum electrostatic calculations based on molecular dynamics free energy simulations. J. Phys. Chem. B 101, 5239–5248 (1997)
Cuello, L. G., Cortes, D. M. & Perozo, E. Molecular architecture of the KvAP voltage-dependent K+ channel in a lipid bilayer. Science 306, 491–495 (2004)
Long, S. B., Campbell, E. B. & MacKinnon, R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science 7 July 2005 (doi:10.1126/science.1116269)
Acknowledgements
We thank M. J. Hahn for technical assistance, M. Holmgren for the cysteine-less Shaker clone, W. Hubell for the gift of dipicrylamine and the members of Bezanilla and Correa laboratories for their comments. This work was supported by funds from an AHA postdoctoral fellowship to B.C., NRSA funding to O.K.A, DFG funding to R.B. and an NIH grant to F.B.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions. The authors declare no competing financial interests.
Supplementary information
Supplementary Discussion
The kinetics and profile of different FRET signals are explained and discussed in detail. Theoretical background and case studies are provided. This file also contains Supplementary Figure Legends. (DOC 48 kb)
Supplementary Figures
This file contains Supplementary Figures S1–S6. Text to accompany these figures is found in the Supplementary Discussion. (PPT 780 kb)
Rights and permissions
About this article
Cite this article
Chanda, B., Kwame Asamoah, O., Blunck, R. et al. Gating charge displacement in voltage-gated ion channels involves limited transmembrane movement. Nature 436, 852–856 (2005). https://doi.org/10.1038/nature03888
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature03888
- Springer Nature Limited
This article is cited by
-
A conformational switch in clathrin light chain regulates lattice structure and endocytosis at the plasma membrane of mammalian cells
Nature Communications (2023)
-
Enlightening activation gating in P2X receptors
Purinergic Signalling (2022)
-
Voltage-dependent gating in K channels: experimental results and quantitative models
Pflügers Archiv - European Journal of Physiology (2020)
-
The HCN channel voltage sensor undergoes a large downward motion during hyperpolarization
Nature Structural & Molecular Biology (2019)
-
Relative positioning of Kv11.1 (hERG) K+ channel cytoplasmic domain-located fluorescent tags toward the plasma membrane
Scientific Reports (2018)