Abstract
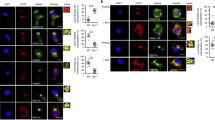
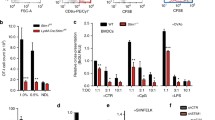
Induction of cytotoxic T-cell immunity requires the phagocytosis of pathogens, virus-infected or dead tumour cells by dendritic cells1. Peptides derived from phagocytosed antigens are then presented to CD8+ T lymphocytes on major histocompatibility complex (MHC) class I molecules, a process called “cross-presentation”2,3. After phagocytosis, antigens are exported into the cytosol and degraded by the proteasome4,5,6. The resulting peptides are thought to be translocated into the lumen of the endoplasmic reticulum (ER) by specific transporters associated with antigen presentation (TAP), and loaded onto MHC class I molecules by a complex “loading machinery” (which includes tapasin, calreticulin and Erp57)7. Here we show that soon after or during formation, phagosomes fuse with the ER. After antigen export to the cytosol and degradation by the proteasome, peptides are translocated by TAP into the lumen of the same phagosomes, before loading on phagosomal MHC class I molecules. Therefore, cross-presentation in dendritic cells occurs in a specialized, self-sufficient, ER–phagosome mix compartment.




Similar content being viewed by others
References
Guermonprez, P., Valladeau, J., Zitvogel, L. & Amigorena, S. Antigen Presentation and T Cell Stimulation by Dendritic Cells. Annu. Rev. Immunol. 20, 620–667 (2002)
Heath, W. R. & Carbone, F. R. Cross-presentation, dendritic cells, tolerance and immunity. Annu. Rev. Immunol. 19, 47–64 (2001)
Yewdell, J. W., Norbury, C. C. & Bennink, J. R. Mechanisms of exogenous antigen presentation by MHC class I molecules in vitro and in vivo: implications for generating CD8 + T cell responses to infectious agents, tumors, transplants, and vaccines. Adv. Immunol. 73, 1–77 (1999)
Kovacsovics-Bankowski, M. & Rock, K. L. A phagosome-to-cytosol pathway for exogenous antigens presented on MHC class I molecules. Science 267, 243–246 (1995)
Norbury, C. C., Chambers, B. J., Prescott, A. R., Ljunggren, H. G. & Watts, C. Constitutive macropinocytosis allows TAP-dependent major histocompatibility complex class I presentation of exogenous soluble antigen by bone marrow-derived dendritic cells. Eur. J. Immunol. 27, 280–288 (1997)
Rodriguez, A., Regnault, A., Kleijmeer, M., Ricciardi-Castagnoli, P. & Amigorena, S. Selective transport of internalized antigens to the cytosol for MHC class I presentation in dendritic cells. Nature Cell Biol. 1, 362–368 (1999)
Cresswell, P., Bangia, N., Dick, T. & Diedrich, G. The nature of the MHC class I peptide loading complex. Immunol. Rev. 172, 21–28 (1999)
Gagnon, E. et al. Endoplasmic reticulum-mediated phagocytosis is a mechanism of entry into macrophages. Cell 110, 119–131 (2002)
Ramachandra, L., Sramkoski, R. M., Canaday, D. H., Boom, W. H. & Harding, C. V. Flow analysis of MHC molecules and other membrane proteins in isolated phagosomes. J. Immunol. Methods 213, 53–71 (1998)
Griffiths, G., Quinn, P. & Warren, G. Dissection of the Golgi complex. I. Monensin inhibits the transport of viral membrane proteins from medial to trans Golgi cisternae in baby hamster kidney cells infected with Semliki Forest virus. J. Cell Biol. 96, 835–850 (1983)
Porgador, A., Yewdell, J. W., Deng, Y., Bennink, J. R. & Germain, R. N. Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity 6, 715–726 (1997)
Lemonnier, F. A. The utility of H-2 class I knockout mice. Virus Res. 82, 87–90 (2002)
Rock, K. L., York, I. A., Saric, T. & Goldberg, A. L. Protein degradation and the generation of MHC class I-presented peptides. Adv. Immunol. 80, 1–70 (2002)
Reits, E. et al. Peptide diffusion, protection, and degradation in nuclear and cytoplasmic compartments before antigen presentation by MHC class I. Immunity 18, 97–108 (2003)
Hogquist, K. A. et al. T cell receptor antagonist peptides induce positive selection. Cell 76, 17–27 (1994)
Winzler, C. et al. Maturation stages of mouse dendritic cells in growth factor-dependent long-term cultures. J. Exp. Med. 185, 317–328 (1997)
Thery, C. et al. Molecular characterization of dendritic cell-derived exosomes: selective accumulation of the heat shock protein hsc73. J. Cell Biol. 147, 599–610 (1999)
Desjardins, M., Huber, L. A., Parton, R. G. & Griffiths, G. Biogenesis of phagolysosomes proceeds through a sequential series of interactions with the endocytic apparatus. J. Cell Biol. 124, 677–688 (1994)
Daniel, S., Caillat-Zucman, S., Hammer, J., Bach, J. F. & van Endert, P. M. Absence of functional relevance of human transporter associated with antigen processing polymorphism for peptide selection. J. Immunol. 159, 2350–2357 (1997)
Karttunen, J., Sanderson, S. & Shastri, N. Detection of rare antigen-presenting cells by the lacZ T-cell activation assay suggests an expression cloning strategy for T-cell antigens. Proc. Natl Acad. Sci. USA 89, 6020–6024 (1992)
Raposo, G., Kleijmeer, M. J., Posthuma, G., Slot, J. W. & Geuze, H. J. in Handbook of Experimental Immunology 5th edn, Ch. 208, 1–11 (Elsevier, Cambridge, MA, 1997)
Houde, M. et al. Phagosomes are competent organelles for antigen cross-presentation. Nature 425, 402–406 (2003)
Acknowledgements
We want to thank S. Hugues for help with the OT-1 effector cells, J. Griffith for technical assistance and A. Lennon-Dusmenil for critical reading of the manuscript. P.G. is a Fellow of the ARC and CNRS. This work was supported by the Institut Curie, the INSERM and the Ligue de Contre le Cancer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Guermonprez, P., Saveanu, L., Kleijmeer, M. et al. ER–phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature 425, 397–402 (2003). https://doi.org/10.1038/nature01911
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature01911
- Springer Nature Limited
This article is cited by
-
Sec22b regulates phagosome maturation by promoting ORP8-mediated lipid exchange at endoplasmic reticulum-phagosome contact sites
Communications Biology (2023)
-
Increased cross-presentation by dendritic cells and enhanced anti-tumour therapy using the Arp2/3 inhibitor CK666
British Journal of Cancer (2023)
-
Lymph node-targeted neoantigen nanovaccines potentiate anti-tumor immune responses of post-surgical melanoma
Journal of Nanobiotechnology (2022)
-
Trinity immune enhancing nanoparticles for boosting antitumor immune responses of immunogenic chemotherapy
Nano Research (2022)
-
The receptor DNGR-1 signals for phagosomal rupture to promote cross-presentation of dead-cell-associated antigens
Nature Immunology (2021)