Abstract
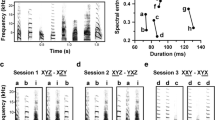
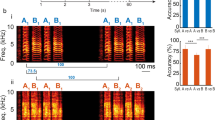
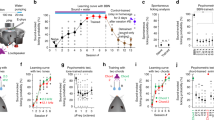
The neural representations associated with learned auditory behaviours, such as recognizing individuals based on their vocalizations, are not well described. Higher vertebrates learn to recognize complex conspecific vocalizations that comprise sequences of easily identified, naturally occurring auditory objects1,2, which should facilitate the analysis of higher auditory pathways. Here we describe the first example of neurons selective for learned conspecific vocalizations in adult animals—in starlings that have been trained operantly to recognize conspecific songs. The neuronal population is found in a non-primary forebrain auditory region, exhibits increased responses to the set of learned songs compared with novel songs, and shows differential responses to categories of learned songs based on recognition training contingencies. Within the population, many cells respond highly selectively to a subset of specific motifs (acoustic objects) present only in the learned songs. Such neuronal selectivity may contribute to song-recognition behaviour, which in starlings is sensitive to motif identity3,4. In this system, both top-down and bottom-up processes may modify the tuning properties of neurons during recognition learning, giving rise to plastic representations of behaviourally meaningful auditory objects.




Similar content being viewed by others
References
Kroodsma, D. E. & Miller, E. H. (eds) The Design of Animal Communication (MIT Press, Cambridge, Massachusetts, 1999)
Gentner, T. Q. & Margoliash, D. in Acoustic Communication (eds Simmons, A. M., Popper, A. N. & Fay, R. R.) 324–386 (Springer, New York, 2002)
Gentner, T. Q., Hulse, S. H., Bentley, G. E. & Ball, G. F. Individual vocal recognition and the effect of partial lesions to HVc on discrimination, learning, and categorization of conspecific song in adult songbirds. J. Neurobiol. 42, 117–133 (2000)
Gentner, T. Q. & Hulse, S. H. Perceptual classification based on the component structure of song in European starlings. J. Acoust. Soc. Am. 107, 3369–3381 (2000)
Kroodsma, D. E. & Miller, E. H. (eds) Ecology and Evolution of Acoustic Communication in Birds (Cornell Univ. Press, Ithaca, 1996)
Eens, M., Pinxten, R. & Verheyen, R. F. Temporal and sequential organization of song bouts in the starling. Ardea 77, 75–86 (1989)
Gentner, T. Q. & Hulse, S. H. Perceptual mechanisms for individual vocal recognition in European starlings, Sturnus vulgaris. Anim. Behav. 56, 579–594 (1998)
Vates, G. E., Broome, B. M., Mello, C. V. & Nottebohm, F. Auditory pathways of caudal telencephalon and their relation to the song system of adult male zebra finches. J. Comp. Neurol. 366, 613–642 (1996)
Müller, C. M. & Leppelsack, H. J. Feature-extraction and tonotopic organization in the avian auditory forebrain. Exp. Brain Res. 59, 587–599 (1985)
Sen, K., Theunissen, F. E. & Doupe, A. J. Feature analysis of natural sounds in the songbird auditory forebrain. J. Neurophysiol. 86, 1445–1458 (2001)
Margoliash, D. Acoustic parameters underlying the responses of song-specific neurons in the white-crowned sparrow. J. Neurosci. 3, 1039–1057 (1983)
Margoliash, D. Preference for autogenous song by auditory neurons in a song system nucleus of the white-crowned sparrow. J. Neurosci. 6, 1643–1661 (1986)
Leppelsack, H. J. & Vogt, M. Responses of auditory neurons in forebrain of a songbird to stimulation with species-specific sounds. J. Comp. Physiol. 107, 263–274 (1976)
Scheich, H., Langner, G. & Bonke, D. Responsiveness of units in the auditory neostriatum of the guinea fowl (numida-meleagris) to species-specific calls and synthetic stimuli, 2: discrimination of iambus-like calls. J. Comp. Physiol. 132, 257–276 (1979)
Wang, X. & Kadia, S. C. Differential representation of species-specific primate vocalizations in the auditory cortices of marmoset and cat. J. Neurophysiol. 86, 2616–2620 (2001)
Rauschecker, J. P., Tian, B. & Hauser, M. Processing of complex sounds in the macaque nonprimary auditory cortex. Science 268, 111–114 (1995)
Gilbert, C. D., Sigman, M. & Crist, R. E. The neural basis of perceptual learning. Neuron 31, 681–697 (2001)
Bakin, J. S. & Weinberger, N. M. Classical conditioning induces CS-specific receptive field plasticity in the auditory cortex of the guinea pig. Brain Res. 536, 271–286 (1990)
Recanzone, G. H., Schreiner, C. E. & Merzenich, M. M. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J. Neurosci. 13, 87–103 (1993)
Kilgard, M. P. & Merzenich, M. M. Order-sensitive plasticity in adult primary auditory cortex. Proc. Natl Acad. Sci. USA 99, 3205–3209 (2002)
Kay, L. M. & Laurent, G. Odor- and context-dependent modulation of mitral cell activity in behaving rats. Nature Neurosci. 2, 1003–1009 (1999)
Kilgard, M. P. & Merzenich, M. M. Plasticity of temporal information processing in the primary auditory cortex. Nature Neurosci. 1, 727–731 (1998)
Logothetis, N. K., Pauls, J. & Poggio, T. Shape representation in the inferior temporal cortex of monkeys. Curr. Biol. 5, 552–563 (1995)
Kobatake, E., Wang, G. & Tanaka, K. Effects of shape-discrimination training on the selectivity of inferotemporal cells in adult monkeys. J. Neurophysiol. 80, 324–330 (1998)
Rainer, G., Asaad, W. F. & Miller, E. K. Selective representation of relevant information by neurons in the primate prefrontal cortex. Nature 393, 577–579 (1998)
Rainer, G. & Miller, E. K. Effects of visual experience on the representation of objects in the prefrontal cortex. Neuron 27, 179–189 (2000)
Tchernichovski, O., Mitra, P. P., Lints, T. & Nottebohm, F. Dynamics of the vocal imitation process: how a zebra finch learns its song. Science 291, 2564–2569 (2001)
Acknowledgements
We thank F. E. Theunissen for generously providing software and assistance for calculating STRFs, M. Konishi and L. M. Kay for valuable critiques of the manuscript, Z. Chi and S. Shea for helpful discussions, and D. Baleckaitis for histology. This work was supported by grants from the National Institutes of Health to T.Q.G. and D.M.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing financial interests.
Rights and permissions
About this article
Cite this article
Gentner, T., Margoliash, D. Neuronal populations and single cells representing learned auditory objects. Nature 424, 669–674 (2003). https://doi.org/10.1038/nature01731
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/nature01731
- Springer Nature Limited
This article is cited by
-
Novel sound exposure drives dynamic changes in auditory lateralization that are associated with perceptual learning in zebra finches
Communications Biology (2023)
-
Multi-channel recordings reveal age-related differences in the sleep of juvenile and adult zebra finches
Scientific Reports (2023)
-
Communication breakdown: Limits of spectro-temporal resolution for the perception of bat communication calls
Scientific Reports (2021)
-
Sleep-dependent reconsolidation after memory destabilization in starlings
Nature Communications (2018)