Abstract
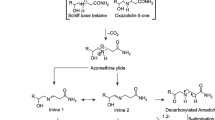
Reports of the presence of acrylamide in a range of fried and oven-cooked foods1,2 have caused worldwide concern because this compound has been classified as probably carcinogenic in humans3. Here we show how acrylamide can be generated from food components during heat treatment as a result of the Maillard reaction between amino acids and reducing sugars. We find that asparagine, a major amino acid in potatoes and cereals, is a crucial participant in the production of acrylamide by this pathway.


Similar content being viewed by others
References
Rosen, J. & Hellenas, K.-E Analyst 127, 880–882 (2002).
Tareke, E., Rydberg, P., Karlsson, P., Eriksson, S. & Törnqvist, M. J. Agric. Food Chem. 50, 4998–5006 (2002).
IARC IARC Monographs on the Evaluation of Carcinogenic Risks to Humans 60, 389 (1994).
Belitz, H.-D & Grosch, W. Food Chemistry (Springer, New York, 1999).
Martin, F. L. & Ames, J. M. J. Agric. Food Chem. 49, 3885–3892 (2001).
Dembinski, E. & Bany, S. J. Plant Physiol. 138, 494–496 (1991).
Castle, L. J. Agric. Food Chem. 41, 1261–1263 (1993).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Mottram, D., Wedzicha, B. & Dodson, A. Acrylamide is formed in the Maillard reaction. Nature 419, 448–449 (2002). https://doi.org/10.1038/419448a
Issue Date:
DOI: https://doi.org/10.1038/419448a
- Springer Nature Limited
This article is cited by
-
Transcriptome analysis of the cerebral cortex of acrylamide-exposed wild-type and IL-1β-knockout mice
Archives of Toxicology (2024)
-
Evaluation of the efficiency of thermostable l-asparaginase from B. licheniformis UDS-5 for acrylamide mitigation during preparation of French fries
World Journal of Microbiology and Biotechnology (2024)
-
Determination of furan and alkylfuran in breakfast cereals from the European market and their correlation with acrylamide levels
European Food Research and Technology (2024)
-
Protective role of vitamin E against acrylamide-induced testicular toxicity from pregnancy to adulthood: insights into oxidative stress and aromatase regulation
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
-
The effect of the use of pekmez and honey as sugar substitutes on the quality characteristics and the acrylamide content of sponge cakes and cookies
Journal of Food Measurement and Characterization (2024)