Abstract
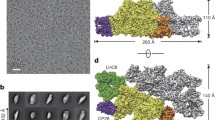
The photosystem II complex, which is the most abundant membrane protein in chloroplasts, comprises the light-harvesting complex II and a reaction-centre core. The reaction centre uses the solar energy collected by the light-harvesting complex II to withdraw electrons from water, releasing oxygen into the atmosphere. It thus generates an electrochemical potential, providing the energy for carbon dioxide fixation and the synthesis of organic molecules, which make up the bulk of the biosphere1. The structure of the light-harvesting complex II has been determined at 3.4-Å resolution by electron crystallography2, but the high-resolution structure of the photosystem II reaction centre and other core components remained unknown. We have grown well-ordered two-dimensional crystals of a sub-core complex containing the reaction centre from spinach thylakoid membranes and used electron crystallography to obtain a projection map of its structure at 8-Å resolution. The features reveal the likely location of the key components that are active in electron transport, and suggest a structural homology and evolutionary links, not only with the purple bacterial reaction centre but also with the reaction centre of photosystem I.









Similar content being viewed by others
References
Barber, J. & Andersson, B. Revealing the blueprint of photosynthesis. Nature 370, 31–34 (1994).
Kühlbrandt, W., Wang, D. N. & Fujiyoshi, Y. Atomic model of plant light-harvesting complex by electron crystallography. Nature 367, 614–621 (1994).
Nanba, O. & Satoh, K. Isolation of a photosystem II reaction center consisting of D-1 and D-2 polypeptides and cytochrome b-559. Proc. Natl Acad. Sci. USA 84, 109–112 (1987).
Barber, J. et al. Characterisation of a PS II reaction centre isolated from the chloroplasts of Pisum sativum. FEBS Lett. 220, 67–73 (1987).
Jansson, S. The light-harvesting chlorophyll a/b -binding proteins. Biochim. Biophys. Acta 1184, 1–19 (1994).
Nakazato, K. et al. Two-dimensional crystallization and cryo-electron microscopy of photosystem II. J. Mol. Biol. 257, 225–232 (1996).
Michel, H. et al. The ‘light’ and ‘medium’ subunits of the photosynthetic reaction centre from Rhodopseudomonas viridis : isolation of the genes, nucleotide and amino acid sequence. EMBO J. 5, 1149–1158 (1986).
Krauss, N. et al. Photosystem I at 4 Å resolution represents the first structural model of a joint photosynthetic reaction centre and core antenna system. Nature Struct. Biol. 3, 965–973 (1996).
Vermaas, W. F. J. Evolution of heliobacteria: implications for photosynthetic reaction center complexes. Photosynth. Res. 41, 285–294 (1994).
Rutherford, A. W. & Nitschke, W. in Origin and Evolution of Biological Energy Conversion, (ed. Baltscheffsky, H.) 143–174 (VCH, New York, (1996)).
Fromme, P. et al. Structure of Photosystem I at 4.5 Å resolution: A short review including evolutionary aspects. Biochim. Biophys. Acta. 1275, 76–83 (1996).
Boekema, E. J. et al. Supramolecular structure of the photosystem II complex from green plants and cyanobacteria. Proc. Natl Acad. Sci. USA 92, 175–179 (1995).
Wang, D. N. & Kühlbrandt, W. High-resolution electron crystallography of light-harvesting chlorophyll a/b -protein complex in three different media. J. Mol. Biol. 217, 691–699 (1991).
Henderson, R., Baldwin, J. M., Downing, K. H., Lepault, J. & Zemlin, F. Structure of purple membrane from Halobacterium halobium : recording, measurement and evaluation of electron micrographs at 3.5 Å resolution. Ultramicroscopy 19, 147–178 (1986).
Henderson, R. et al. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J. Mol. Biol. 213, 899–929 (1990).
Frank, J., Shimkin, B. & Dowse, H. SPIDER-A modular software system for electron image processing. Ultramicroscopy 6, 343–358 (1981).
Acknowledgements
We thank G. Friso for her involvement in the project, W. Schubert for supplying PSI data, and E. Orlova for advice on alignment by cross-correlation. This work was supported by the Biotechnology and Biological Sciences Research Council (J.B.) and by a fellowship to K.-H.R. from the Boehringer Ingelheim Fonds.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rhee, KH., Morris, E., Zheleva, D. et al. Two-dimensional structure of plant photosystem II at 8-Å resolution. Nature 389, 522–526 (1997). https://doi.org/10.1038/39103
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/39103
- Springer Nature Limited
This article is cited by
-
Structural roles of lipid molecules in the assembly of plant PSII−LHCII supercomplex
Biophysics Reports (2018)
-
One-step isolation and biochemical characterization of a highly active plant PSII monomeric core
Photosynthesis Research (2011)
-
Crystallization of the Photosystem II core complex and its chlorophyll binding subunit CP43 from transplastomic plants of Nicotiana tabacum
Photosynthesis Research (2010)
-
Spectral hole burning: examples from photosynthesis
Photosynthesis Research (2009)
-
Photosystem II: Structure and mechanism of the water:plastoquinone oxidoreductase
Photosynthesis Research (2007)