Abstract
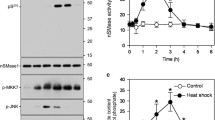
THE sphingomyelin pathway, initiated by hydrolysis of sphingo-myelin to ceramide and stimulation of a Ser/Thr ceramide-activated protein (CAP) kinase, mediates tumour necrosis factor-α (TNF-α) and interleukin-1β action1–4. CAP kinase is membrane-bound and proline-directed, recognizing the minimal substrate motif Thr-Leu-Pro (ref. 5). TNF may use the sphingomyelin pathway to signal Raf1 to activate the MAP kinase cascade6–8. Evidence shows that cytoplasmic Rafl binds to GTP-ras upon cellular stimulation, is recruited to the plasma membrane, and activated9,11. How membrane-bound Rafl is activated is uncertain, but regulation of its kinase activity may involve its phosphorylation12–19. Specific Raf kinases, however, have not hitherto been identified. Here we report that CAP kinase phosphorylates Rafl on Thr 269, increasing its activity towards MEK (MAP kinase or ERK kinase). Moreover, in intact HL-60 cells, CAP kinase complexes with Rafl and, in response to TNF and ceramide analogues, phosphorylates and activates Rafl, implicating CAP kinase as a link between the TNF receptor and Rafl.
Similar content being viewed by others
References
Kolesnick, R. & Golde, D. W. Cell 77, 325–328 (1994).
Liu, J., Mathias, S., Yang, Z. & Kolesnick, R. N. J. biol. Chem. 269, 3047–3052 (1994).
Hannun, Y. A. J. biol. Chem. 269, 3125–3128 (1994).
Heller, R. A. & Kronke, M. Cell Biol. 126, 5–9 (1994).
Joseph, C. K., Byun, H.-S., Bittman, R. & Kolesnick, R. N. J. biol. Chem. 268, 2002–2006 (1993).
Belka, C. et al. EMBO J. 14, 1156–1165 (1995).
Raines, M. A., Kolesnick, R. N. & Golde, D. W. J. biol. Chem. 268, 14572–14575 (1993).
Vietor, I., Schwenger, P., Li, W., Schlessinger, J. & Vilcek, J. J. biol. Chem. 268, 18994–18999 (1993).
Zhang, X.-F. et al. Nature 364, 308–313 (1993).
Stokoe, D., MacDonald, S. G., Cadwallader, K., Symons, M. & Hancock, J. F. Science 264, 1463–1467 (1994).
Leever, S. J., Paterson, H. F. & Marshall, C. J. Nature 369, 411–414 (1994).
Daum, G., Eisenmann-Tappe, I., Fries, H.-W., Troppmair, J. & Rapp, U. R. Trends biochem. Sci. 19, 474–480 (1994).
Williams, N. G. & Roberts, T. M. Cancer Metastasis Rev. 13, 105–116 (1994).
Kyriakis, J. M., Force, T. L., Rapp, U. R., Bonventre, J. V. & Avruch, J. J. biol. Chem. 268, 16009–16019 (1993).
Kovacina, K. S. et al. J. biol. Chem. 265, 12115–12118 (1990).
Morrison, D. K. et al. Cell 58, 649–657 (1989).
Kolch, W. et al. Nature 364, 249–252 (1993).
Morrison, D. K., Heidecker, G., Rapp, U. R. & Copeland, T. D. J. biol. Chem. 268, 17309–17316 (1993).
Dent, P., Jelinek, T., Morrison, D. K., Weber, M. J. & Sturgill, T. W. Science 268, 1902–1906 (1995).
Macdonald, S. G. et al. Molec. cell. Biol. 13, 6615–6220 (1993).
Izumi, T. et al. J. biol. Chem. 266, 7933–7939 (1991).
Pantazis, P., Kharbanda, S., Goustin, A. S., Galanopoulos, T. & Kufe, D. Proc. natn. Acad. Sci. U.S.A. 88, 2481–2485 (1991).
Wartmann, M. R. & Davis, R. J. biol. Chem. 269, 6695–6701 (1994).
Fantl, W. J. et al. Nature 371, 612–614 (1994).
Freed, E., Symons, M., Macdonald, S. G., McMormick, F. & Ruggieri, R. Science 265, 1713–1716 (1994).
Fu, H. et al. Science 266, 126–129 (1994).
App, H. et al. Molec. cell. Biol. 11, 913–919 (1991).
Wang, H.-G. et al. Oncogene 9, 2751–2756 (1994).
Guilbins, E. et al. Immunity 2, 341–351 (1995).
Morre, D. J. et al. Meth. Enzym. 228, 448–450 (1994).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yao, B., Zhang, Y., Delikat, S. et al. Phosphorylation of Raf by ceramide-activated protein kinase. Nature 378, 307–310 (1995). https://doi.org/10.1038/378307a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/378307a0
- Springer Nature Limited
This article is cited by
-
The role of CRAF in cancer progression: from molecular mechanisms to precision therapies
Nature Reviews Cancer (2024)
-
A novel mechanism of ERK1/2 regulation in smooth muscle involving acetylation of the ERK1/2 scaffold IQGAP1
Scientific Reports (2017)
-
Ras signaling in NGF reduction and TNF-α-related pancreatic β cell apoptosis in hyperglycemic rats
Apoptosis (2012)
-
Ceramide triggers an NF-κB-dependent survival pathway through calpain
Cell Death & Differentiation (2005)